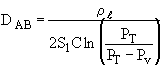Chapter 14: External Diffusion Effects on Heteregeneous Reactions
Example: Measurement of Gas Phase Diffusivities
Consider the test tube partially filled with a volatile liquid shown below.
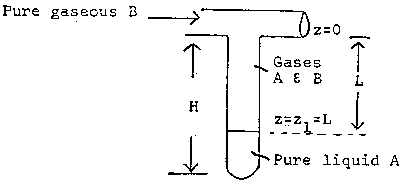
A mass balance on A in the gas phase gives

Using the quasi-state assumption that the concentration at each point z changes very slowly with time,
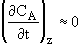
Then
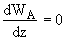
Integrating WA = Number which is independent of distance, z, but is a function of time, t
WA = f(t)
For diffusion of A through a stagnant gas
WB = 0
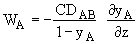
at z = 0 yA = yA2 = 0
z = zo
= L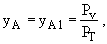
where PV = vapor pressure and PT = total pressure
Since NA is independent of z under the quasi-steady assumption, the above differential equation can be integrated to give
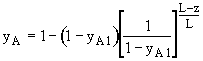
Then
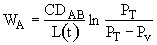
For constant temperature and pressure, C = total concentration = a
constant = . Only the distance between the top of the capillary and the gas-liquid
interface is varying.
. Only the distance between the top of the capillary and the gas-liquid
interface is varying.
Overall balance on the entire system
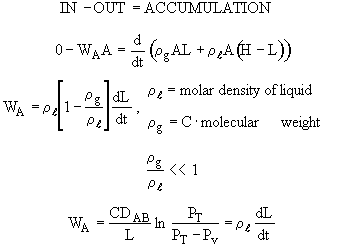
Initial conditions: t = 0 , L = Lo
Integrating
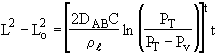
Rearranging:
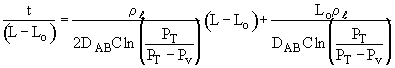
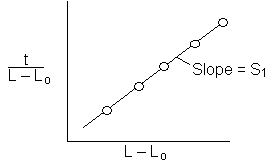
The height of the gas-liquid interface, L, can easily be measured with a cathetometer. A plot at t/L–Lo vs. (L–Lo) should yield a straight line of slope S from which the diffusivity can be calculated from the equation