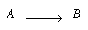Chapter 16: Distributions of Residence Times for Chemical Reactors
Additional Homework Problems
WEB-P16-1B
| The third-order gas-phase reaction | ||
|
|
||
is to be carried out in a nonideal reactor whose macromixing
can be characterized by the RTD in Figure 13-9(a). The space-times corresponding
to the figure are s
and the entering concentration of A is 0.02 mol/dm3.
Calculate the conversion assuming that the micromixing is described by: s
and the entering concentration of A is 0.02 mol/dm3.
Calculate the conversion assuming that the micromixing is described by:
(a) Figure E13-3.1. (b) Figure E13-3.2. (c) Three reactors in series: a PFR (  = 25 s) followed
by a CSTR ( = 25 s) followed
by a CSTR ( = 50 s) and then a PFR ( = 50 s) and then a PFR ( = 25
s). = 25
s).(d) The complete segregation and maximum mixedness models. |
[2nd Ed. P13-10]