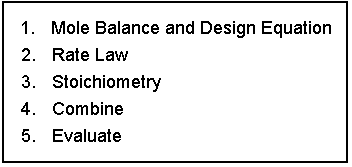
French Menu Analogy


Example: The elementary gas phase reaction
takes place in a CSTR at constant temperature (500 K) and constant pressure (16.4 atm). The feed is equal molar in A and B.
Mole Balance Rate Law Stoichiometry gas phase, isothermal (T = T0), no pressure drop (P = P0) 
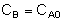



[Why do you suppose CB is a constant, when B is being consumed?] Combine Evaluate
of this reaction carried out in a PFR and a Batch Reactor.
Example: The elementary liquid phase reaction
is carried out isothermally in a CSTR. Pure A enters at a volumetric flow rate of 25 dm3/s and at a concentration of 0.2 mol/dm3.
What CSTR volume is necessary to achieve a 90% conversion when k = 10 dm3/(mol*s)?
Mole Balance Rate Law 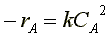
Stoichiometry liquid phase (v = vo) 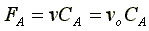
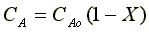


Combine Evaluate at X = 0.9,
V = 1125 dm3 Space Time