Analyze the following second order gas phase reaction that occurs isothermally in a PBR:
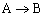
Analyze the following second order gas phase reaction that occurs isothermally in a PBR:
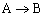
Mole BalanceMust use the differential form of the mole balance to separate variables:

Rate LawSecond order in A and irreversible:
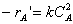
Stoichiometry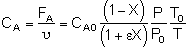

Isothermal, T = T0
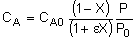
Combine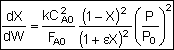



Need to find (P/P0) as a function of W (or V if you have a PFR).
Ergun Equation Variable Density 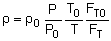


let Catalyst Weight where 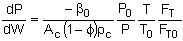


let then 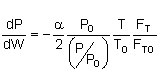
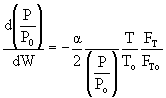



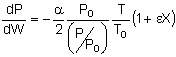


Isothermal Operation let then recall that notice that The two expressions are coupled ordinary differential equations. We can solve them simultaneously using an ODE solver such as Polymath. For the special case of isothermal operation and epsilon = 0, we can obtain an analytical solution.
Polymath will combine the mole balance, rate law and stoichiometry.
Analytical Solution
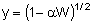


Combine Solve 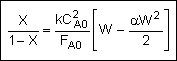

Could now solve for X given W, or for W given X.
Pressure and Reaction Orders
We want to learn how the various parameters (particle diameter, porosity, etc.) affect the pressure drop and hence conversion. We need to know how to respond to "What if…" questions, such as:
"If we double the particle size, decrease the porosity by a factor of 3, and double the pipe size, what will happen to D P and X?"
To answer these questions we need to see how a varies with these parameters.
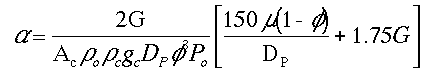
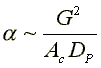

Compare Case 1 and Case 2:
For example, Case 1 might be our current situation and Case 2 might be the parameters we want to change to.
For constant mass flow through the system
= constant
Laminar Flow
Effect of Reducing Particle Size on Conversion in a PBR
POLYMATH
Consider the following gas phase reaction carried out isothermally in a packed bed reactor. Pure A is fed at a rate of 2.5 moles/s and with
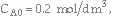
, and a = 0.0002 kg-1.
2A
B
Mole Balance
Elementary
Rate Law
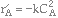
Stoichiometry
Gas with T = T0
A
B/2
POLYMATH will combine everything
________________
Profiles
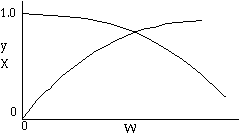
"What Four Things are Wrong with this Solution?" (Chapter 4)
| Mole Balance | 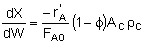 |
|
| Stoichiometry | 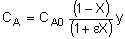 |
|
| Cross-sectional Area | 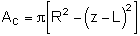 |
|
| Pressure Drop Equations | 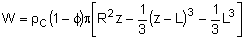 |
|
 |
||
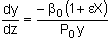 |
||
 |
||
| Combine | Polymath will combine for you |