Asynchronous Learning of Chemical Reaction Engineering
Neelesh Varde[1] and H. Scott Fogler[2]
Department of Chemical Engineering
The University of Michigan
Ann Arbor, Michigan 48109-2136
Abstract
In the last five years, there have been significant advances in
technology, such as the widespread use of the Internet. With this technological
progress, education has begun to move forward as well, to better accommodate the
diverse needs of students. With this concept in mind, we explore the idea of
Asynchronous Learning (AL). AL is based on the idea that students can learn
course material at different times and locations, in contrast to synchronous
learning, in which students learn by attending a conventional lecture or
laboratory. The asynchronous learning environment provides students with the
teaching materials and tools for registration, instruction, and discussion,
however; there are no lectures. As a result, the students continually
communicate with the professor and the TA through e-mail. The key to a
successful AL course is to provide students with material with which they can
continually interact.
Seven students took the Principles of Chemical Reaction Engineering
course, ChE 344, asynchronously during the summer of 2000 and all students
successfully completed the course. An additional nine students are taking the
course during summer 2001. Interactive CDs containing summary notes with audio
clips and self tests, interactive computer modules, and example problems were
given to the students as a supplement to the textbook. There was also a course
website containing unit descriptions, class information, course grades, a
bulletin board, and updates. Traditionally a four credit hour class for junior
level students, the course was broken down into 21 units of reading assignments
and homework. Additionally, students completed an open-ended project (OEP) to
further explore chemical reaction engineering principles, and took two mid term
exams and a final exam under the observation of a proctor. A teaching assistant
checked his e-mail throughout the day and early evening to respond to the
student’s questions.
Some of the most important positive outcomes of the course were that the responsibility for learning the material was transferred from the professor to the students and that students stated that they felt more self confident after having taken the course. The downside was the students missed face-to-face interaction with the professor, teaching assistant and other students.
Introduction
With the emergence and widespread use of computers during the past twenty
years, technology has progressed further than many ever thought possible. These
advancements have had significant impacts on education. Through ever increasing
technological advancements, education has expanded to better meet the diverse
needs of students. An excellent review of the literature by Kadiyala and Crynes
provides evidence that instructional technology enhances learning.[i]
With advances in instructional technology, a variety of student learning styles
described by the Felder and Soloman[ii] Inventory (e.g., active,
reflective, global, sequential) can be addressed, thereby reducing the need for
a synchronous course with a lecture. Wallace and Mutooni,[iii] Felder and Brent,[iv] discuss these
advantages.
Asynchronous Learning (AL) is the concept that students can learn at
different locations and at different times. AL is opposite to synchronous
learning, where students learn at the same time and place in traditional
activities such as classroom lecture and laboratory sessions. Recently, Dutton
et.al. showed that on-line AL students in their course performed better than the
lecture students.[v]
The asynchronous learning environment provides students with interactive
teaching materials and tools for registration, instruction, and discussion.
Student-to-student interaction is provided by a common “conference room”, either
an online chat room, bulletin board, or e-mail group that allows everyone to
post a message, read a message, or respond to a message, all within the same
shared space. Student-faculty/teaching assistant interactions were primarily
through e-mail with an occasional phone call.
Technology has facilitated the use of AL and made it a viable alternative
to synchronous learning. Now with widespread Internet use, as well as faster
connections and more powerful computers, it is easy to provide interactive
lessons. Students can communicate with other students, read and interact with
the course summary notes on the web, and even check their own grades.
Because AL involves the ability to maintain communication without having to meet at the same place at the same time, students who work during the day or have family responsibilities at home can easily take a class, without having to worry about commuting to a college or university at night. Another benefit is that because AL involves self-paced study, a student who has important priorities one week, can easily move back coursework to a more convenient time. However, if the teaching assistant finds the students are falling too far behind they will receive a prod, usually through e-mail. Because of these benefits, AL has emerged as a popular alternative to many students.
Course Content
The University of Michigan’s Chemical Engineering 344 is entitled Principles of Chemical Reaction
Engineering. This course covers the fundamentals of chemical reaction
engineering including: rate laws, kinetics, mechanisms of homogeneous and
heterogeneous reactions, analysis of rate data, multiple reactions, adiabatic
and non-adiabatic reactors, safety, and multiple reactions with heat effects.
Emphasis is placed on logic rather than
memorization of equations and the conditions to which they apply.
Course Structure
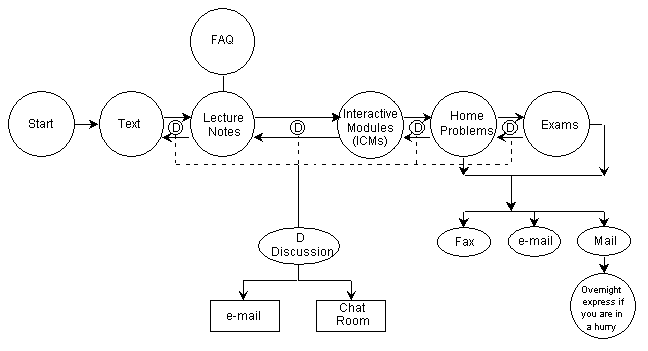
The class, normally a 4-credit course for junior level students, was divided into 21 self-paced units. Each of these units contains a textbook and CD reading assignment, mandatory homework problems, recommended study problems, and solved problems. In addition to the 21 units, students must take two tests and a final exam, and complete an open-ended project (OEP).

Figure 1. Organizational Structure of the Course
Class Resources
ChE 344 was the first class chosen in the department of chemical engineering to be offered through asynchronous learning because of the enormous course resources that had been built up over the years. In addition to the textbook, Elements of Chemical Reaction Engineering, 3rd Edition by H. Scott Fogler[vi] each student is provided with an interactive CD and the class web page (URL: http://www.engin.umich.edu/~cre/asyLearn or CRE URL: http://www.engin.umich. edu/~cre). The interactive CD includes:
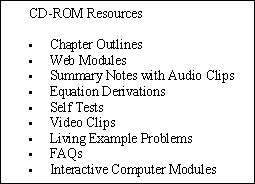
The Chapter Outlines (Figure 2) allow the user an easy index to “surf” the CD ROM. The Summary Notes (Figure 3), with their numerous derivations, examples, links, self-tests (Figure 4), and audio clips (in both wave and mp3 format) are a nice supplement to the text material and are ideal not only for the global learner, but also for the active and sequential learners. Because the questions asked by the students from year-to-year are very similar (if not the same), one of the key ingredients for a successful AL course is the collection and display of these frequently asked questions (FAQs). The FAQs (Figure 5) section provides answers to the most commonly asked questions in previous classes.
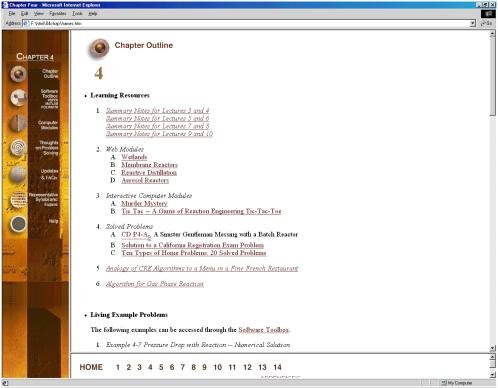
Figure 2. Chapter Outlines
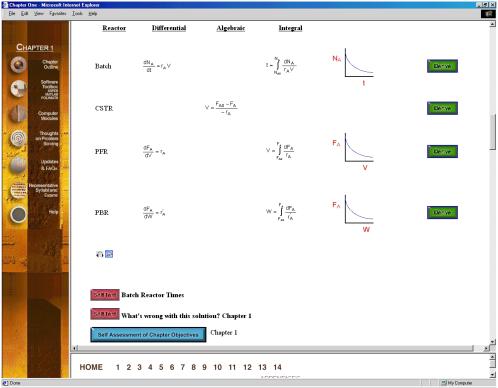
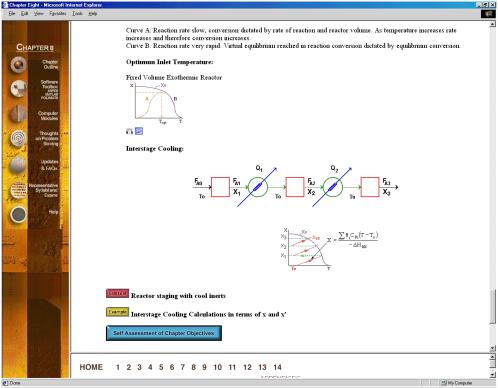
Figure 3. Summary Notes
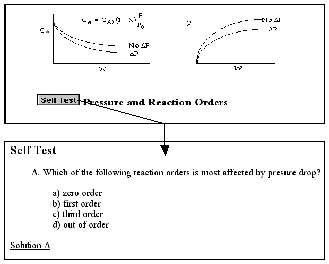

Figure 4.
Self-Tests
Figure 5. FAQs
Simulations are also a major component of the CD, as web modules, interactive computer modules (ICMs) (Figure 6) and living example problems are included as well. The web modules (Figure 7) are stand-alone lessons that show novel applications of chemical reaction engineering principles. Each ICM has a description of the module, a review of the fundamentals, and an interactive scenario on which the students are graded by the computer (Figure 8). The living example problems are a new concept. Here, the examples in the textbook are also on the CD ROM so that they can easily be loaded onto the students’ computer. Living example problems are designed for students to easily vary parameters in the text example problems in order to understand real life problems and ask “what if” questions that allow them to practice their creative and critical thinking skills. The eight ICMs and six Web Modules are well suited for active and sequential learners. The ICMs allow students to ask “what if” questions as well as enjoy practicing reaction engineering concepts, while the Web Modules enable students to learn how reaction engineering principles can be applied to a variety of real world situations. The CD also features a Professional Reference Shelf, which includes material important to the practicing engineer, which is typically not included in the majority of chemical reaction engineering courses.
Figure 6. Living Example
Problems
Simulations

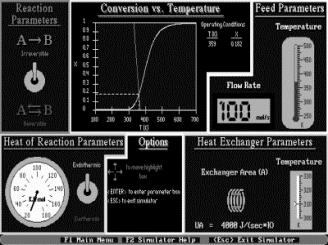
Figure 7. Web Modules
Figure 8. ICMs
Addressing Different Student Learning Styles
Research has shown that not everyone learns the same way. One of the more cited ways to classify the different learners is that given by Felder and Soloman.

Virtually all the different learning styles are addressed in the
resources available for the AL course. For example, the global learner can
obtain an overview of the material from the web Summary Notes before diving into
the text for the details. The sequential learner can interact with the Derive
hot buttons to see the details of the derivation of an equation. Owning to the
large number of hot buttons (![]() ,
, ![]() ,
, ![]() , and
, and ![]() ) the active learner is continually able to
participate in the learning process. The reflective learner style is addressed
through the self-tests and the ICMs multiple choice quizzes where he/she has a
chance to pause and think about an answer before proceeding further. The visual
learner is able to follow the trends through plotting the variables from the
solutions to Polymath living example problems. The audio clips
) the active learner is continually able to
participate in the learning process. The reflective learner style is addressed
through the self-tests and the ICMs multiple choice quizzes where he/she has a
chance to pause and think about an answer before proceeding further. The visual
learner is able to follow the trends through plotting the variables from the
solutions to Polymath living example problems. The audio clips ![]() in the summary notes, which are
more related to short “sound bytes” rather than reading of the text material,
are a welcome resource to the verbal learner, as is the textbook material.
in the summary notes, which are
more related to short “sound bytes” rather than reading of the text material,
are a welcome resource to the verbal learner, as is the textbook material.
Developing Critical Thinking Skills
Thoughts on critical thinking were taken from R. W. Paul’s book, Critical Thinking[vii] and from the Oklahoma State University Phillips Lecture in April, 1997.[viii] A number of assignments required that the students write a question about the homework problem that required critical thinking and explain why it involved critical thinking. Specifically, Paul’s 6 types of Socratic questions were used. The questions were then collected, and e-mailed back to the students who were asked to vote on the best critical thinking question and make a statement why they felt it was the best. Seeing, judging and comparing other students questions further develop their critical thinking skills.
Developing Creative Thinking Skills
In accordance with ABET requirements, there is an open ended project (OEP) involved in this class. The purpose of the OEP is to give each member of the class a chance to practice and develop their creative thinking skills. To do this students will need to learn and bring creative thinking skills (Osborn’s Vertical Thinking, Futuring, Analogy, DeBono’s Lateral Thinking) to bear on a specific problem, (Fogler and LeBlanc.[ix]).This semester’s OEP consists of the creation of a presentation with Microsoft PowerPoint or equivalent software. The specific topics chosen were researched independently in groups of 2. These topics represent situations that can be modeled using the principles of chemical reaction engineering learned in this course. This type of modeling will include reactor schemes, mathematical models, evaluation of constants, analysis of assumptions, etc. Resources that can be utilized include web sites, journals, books, and class materials. The topics for the Summer 2000 OEP included:
·
“Drinking and Driving” ( a study of alcohol uptake by body),
·
“The Poison Bite Problem” (a study of venom and anti-venom effect
of a poisonous snake)
·
“The Antibiotic Model” (a study concerning antibiotic
concentration in blood with bacterial growth and death).
The students divided almost equally between the “Drinking and Driving” and “The Poison Bite Problem.” The “Drinking and Driving Problem” is currently being developed into a full web module.
Student Perspective
AL is also advantageous from the students’ perspective. First, the course structure of the chemical engineering department is oriented such that certain required classes are only offered every other semester. Because of this arrangement, a student who accepts a co-op job assignment is often at a disadvantage, taking an extra semester to a year to graduate. Secondly, there are also students who may barely fail a course, but who are ready to take it again immediately. AL fulfills this need, recognizing that a student who is fresh with the material is more likely to do better than a student who has been away from the subject matter for a while. Finally, we realized that a lot of students have many obligations (family, work, etc.) that do not allow them the ability to meet for a class regularly. With AL, these students can study when they have free time and don’t have to worry about missing an important lecture or lab session.
Course Logistics
With students taking ChE 344 asynchronously, there are many aspects of the class that have to be logistically considered. These include submitting assignments, taking the exams, getting course material questions answered, and the nature of the open-ended project.
Assignment Submission - Home Problem Assignments were submitted in three ways: by fax, mail, or e-mail as a graphics attachment (a gif or jpg image file). The most popular choice to turn in assignments was submission by e-mail. Here the hand written homework solutions were scanned and attached to an e-mail message to the teaching assistant (TA). The assignments were graded in the normal way and returned to the students.
Exams - Two exams and a final were
required for the class. Because students were scattered throughout the United
States, a proctor system was developed. Students were allowed to take exams only
under the supervision of a proctor. The proctor had to be either a supervisor at
work, another college professor, or a high school teacher. The exams are mailed
to the proctor and returned by the proctor to the TA or professor. The proctor
must sign a statement indicating that he/she monitored the test at all times,
and had not observed any violations of the University of Michigan, College of
Engineering’s honor code.
Getting Questions Answered - Students will inevitably have questions regarding homework assignments and conceptual understanding of course material. They are requested to first read through the FAQs related to the chapter they are studying. For this reason, a teaching assistant (TA) is “on e-mail call” for most of the day, so questions can be answered with a minimum amount of turnaround time, usually less than a day. On the average the TA received about 10-15 e-mails a week. So far, this seems to work. In addition, students have the opportunity to send questions to the class e-mail list.
Grading
Grading in this course, whether synchronous or asynchronous, has always been on a straight scale basis as shown below.
|
A |
90-100 |
|
B |
80-89 |
|
C |
70-79 |
|
D |
66-70 |
|
E |
Below 66 |
The weighting of each component is as follows below.
|
Homework |
20% |
|
OEP |
5% |
|
Comprehensive Problem |
5% |
|
Exam I |
20% |
|
Exam II |
25% |
|
Final Exam |
25% |
|
Total |
100% |
The comprehensive problem is a specific problem in the textbook, which encompasses one of the main goals of the class, to solve a chemical reaction-engineering problem involving multiple reactions with heat effects. Usually either problem P8-29, P8-30, or similar are assigned the comprehensive problem.
Barriers
There are many advantages of an asynchronous class, but there are many barriers as well. A few of these barriers, we were able to remedy, but others really have no apparent solution.
One easily solved barrier related to the web page. The course web page has links to the overall chemical reaction engineering home page, which we initially suggested they visit frequently. The problem was that students using a modem were having problems listening to the audio files, as they were rather large to download (about one megabyte each). To compensate for this, we supplied students with the updated CD containing audio files, which removed the bottleneck (download time) from the studying and learning process. We also made the asynchronous learning course home page very easily downloadable, with no large image files.
Another potential barrier we were able to solve was with students asking questions. We knew that students would have certain times when they would work on homework and reading. If they encountered a frustrating concept or question, we knew that if they felt they could e-mail the TA and get a quick response, that they would be more likely to keep working on it that same period.
Perhaps the biggest barrier that is common in AL courses is student self-discipline. Without any specific deadlines, it is human nature to put off studying and learning until the last possible moment. With one month gone in the course, only two students out of seven were keeping pace with the homework submission. To combat this in the next AL offering of ChE 344, we will implement three or four deadlines for homework. We have already placed deadlines for taking the first test and believe that a few more deadlines would help students stay on track for the course.
Student Profile
Summer 2000
Seven students were enrolled in the course. All seven students completed
the course and passed. Six students were U of M students and one was a junior
ChE student from Northwestern University. Five students were off campus at their
co-op work or summer internship and only two students were on the Ann Arbor
campus. Four of the students took the class because they were required to repeat
the course based on their grade from the previous term. The final grades in the
AL course coincided with the students GPA’s from
previous courses at the university. The final GPA for the course was a 3.04 (2
A’s, 1 B+, 2 B-‘s, 2 C+’s).
Summer 2001
Nine students are enrolled in the course, one of which is out of the country. Based on the progress of the students in the Summer 2000 course the time lines for completing the various units were revised, an additional exam was set, and the window for taking the exams was specified.
Outcomes
At the end of the course the students were asked to fill out a questionnaire/evaluation of the course. In addition, one-on-one interviews were carried out during the Fall term a month or so after the course had been completed. Based on the interviews, questionnaires, exams and exam scores, there appeared to be no significant difference between the seven students who took the course asynchronously during the summer of 2000 and the 135 students who took the course synchronously winter term 2000. One of the major realizations by AL students was that they recognized the responsibility for learning the material was transferred from the instructor to themselves. This realization is desired in every course, not just courses offered asynchronously, in order to help the students develop life long learning skills. Some AL students said their self-confidence increased as a result of successfully completing the course. All students liked the flexibility of the AL course and that it was offered during the summer. Two students commented that during the winter term in which they were in cooperative learning groups, they felt rushed by the other group members and were under stress to understand the material, while the summer 2000 course was virtually all self paced. The next time the course is offered we plan to put deadlines for taking the exams at specified times during the term. In addition, to help the students proceed at a reasonable pace to keep up with the material two or three cumulative deadlines may be imposed. All of the students appreciated the resources available to them, namely the Interactive Computer Modules, Summary Notes with “Derive” hot buttons, extra examples, audios, and self tests. The key goals of a successful AL course are to:
• provide a variety of learning resources to accommodate the different learning styles described by Felder and Soloman.
• keep the student interacting with the computer and the material (hot buttons, simulations).
• provide a number of FAQs collected from previous courses. The AL instructor is not immediately available and a number of the same questions come up year after year.
• help the student reach the realization that the responsibility of learning material is on him/her. There is no instructor around to ask a question after class
• provide incentives that keep the students on the time line.
The negatives of the course one can readily guess, the main one being the lack of face to face interaction between student, faculty, and graduate student instructors, and other students. The chat room/bulletin board was not effective, perhaps in part because of the small number of students. Future AL courses may use professional software to facilitate a “real time” chat room with on-line teaching assistants and professor’s office hours. With respect to the open-ended problems (OEP), generating and developing ideas proved to be quite difficult solely through e-mail and telephone conversations. Only one or two of the OEPs were of above average quality. If students could not find the answers to a question in the FAQ’s, sometimes they had to wait for a few hours if the TA had just checked his e-mail before they signed on. One other drawback was that some of the questions were difficult to explain using e-mail especially those where sketches were required.
A teacher can make every effort to motivate students to learn, but in the end it is the student who is responsible for learning the course material. Asynchronous learning does place a greater responsibility on the student to learn, as compared with a traditional class, but we believe this is good practice for the workplace. When students move onto industrial jobs, there will be many times where they will have to assume the full responsibility of learning. Completing an AL course prepares students for this environment, as well as giving them the confidence that they can learn on their own. In the exit survey of the students, most of them cited their increased self-confidence level as one of the greatest positives in the AL course.
Summary
Chemical reaction engineering was taught asynchronously using e-mail, the web, text and CD-ROM. The wide variety of resources provided to the students allowed for most all of the learning styles described by Felder and Soloman. The students in the course performed well in the AL course and enjoyed it. The two major advantages of the chemical reaction course were that it addressed a number of the learning styles identified in Soloman/Felder’s inventory and that it provided great flexibility in the time and location to learn chemical reaction engineering. The more positive effects were that the students developed a greater sense of self confidence and the realization that the responsibility for learning was transferred from the professor to the student. The major drawback was the lack of face-to-face communication between student, GSIs and faculty. After reviewing the course structure and outcomes from summer 2000, the chemical engineering department’s curriculum committee voted to accept the asynchronous learning version of the chemical reaction engineering, ChE 344, as equivalent to the synchronous version of the course offered during the academic year and it is currently being offered this summer.
[1] Present address: Department of Chemical Engineering, University of Illinois, Urbana IL 61801.
[2] To whom correspondence should be addressed.
Literature Cited
[i] Kadiyala, M. and B. L. Crynes, “A review of the literature on Effectiveness of use Information Technology,” J. of Engineering Education 89, p.177 (2000).
[ii] Felder and Soloman, http://www2.ncsu.edu/unity/lockers/users//f/felder/public/ ILSdir/styles.htm
[iii] Wallace, D.R. and P Mutooni, “A Comparative Evaluation of World Wide Web-Based and Classroom Teaching,” J. Engrg. Edu. 86 p211 (1997).
[iv] Felder, R and R. Brent, “Is Technology a Friend or Foe of Learning,” Chem. Engrg. Edu. 34 p326 (2000).
[v]Dutton, J., M. Dutton, and J. Perry, “Do On-line Students Perform as Well as Lecture Students,” J. of Engineering Education, 90, p.131 (2001).
[vi] The Elements of Chemical Reaction Engineering, 3rd Ed., 959 pages, Prentice Hall, 1999.
[vii] Paul, R. W. Critical Thinking Foundation for Critical Thinking, Santa Rosa, CA, 1995.
[viii] Fogler, H. S., “Teaching Critical Thinking, Creative Thinking, and Problem Solving in the Digital Age,” Phillips Lectureship, Oklahoma State University, April, 1997.
[ix] Fogler, H. Scott and Steven E. LeBlanc, Strategies for Creative Problem Solving, 203 pages, Prentice Hall, 1995.