![]()
|
|
Type 2 Home Problem
Problems that require intermediate calculations or manipulations.
The reaction rate constant, k, is dependent on temperature according to the Arrhenius equation:
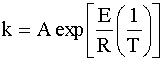
Since the PFR is at a different temperature than the CSTR, we need to relate the two rate constants. The easiest way to do this is to divide the second rate constant by the first rate constant:
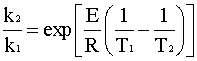
Rearranging for k2 gives:
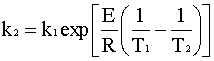
![]() Back to the Solution
Back to the Solution
CRE Thoughts Ten Types Homogeneous Example 2