ASPEN PLUS™ Example Problems
This section is a tutorial to walk you through Problem 11-3 for the 1st edition of Essentials of Chemical Reaction Engineering. You can download the ASPEN backup file here that completes this problem.
Example 11-3 Adiabatic Liquid-Phase Isomerization of Normal Butane
Problem Description
Normal butane, C4H10, is to be isomerized to isobutane in a plug-flow reactor. This elementary reversible reaction is to be carried out adiabatically in the liquid phase under high pressure using a liquid catalyst which gives a specific reaction rate of 31.1 h-1 at 360 K. The feed enters at 330 K.
- Calculate the PFR volume necessary to process 100,000 gal/day (160 kmol/h) at 70% conversion of a mixture 90 mol % n-butane and 10 mol % of i-pentane, which is considered an inert.
- Plot and analyze X, Xe, T and -rA down the length of the reactor
- Calculate the CSTR volume for the same conditions as the PFR.
Components
Three components are considered in the Aspen model: C4H10 (n-butane), IC4H10 (isobutane) and IPENTANE (2-methyl-butane). The liquid catalyst is not included because its flowrate is not known and the specific reaction rate has been given for the reaction condition. These three components are called directly from built-in Aspen pure component databanks.
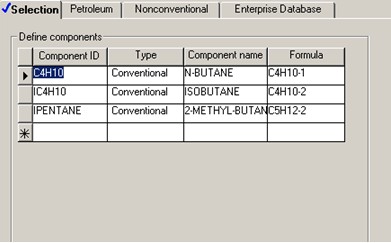
Properties
Different property models can yield different predictions for various thermophysical properties used in mass and energy balance calculations. PENG-ROB, Aspen Peng-Robinson equation-of-state property model, is chosen to describe the thermophysical properties of this hydrocarbon liquid mixture. One of several equations-of-state well-known to be suitable for hydrocarbon systems, Peng-Robinson equation-of-state should provide reasonable calculations for heats of reaction and heat capacities.
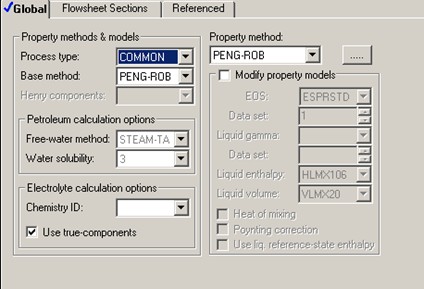
Within the temperature range of 330 K to 360 K, PENG-ROB predicts liquid heat capacity of 157-185 J/mol•K for C4H10, 161-214 J/mol•K for IC4H10, and 176-195 J/mol•K for IPENTANE. The higher the temperature, the higher the heat capacity will be. Pressure also affects liquid heat capacity. The predictions here are done at 1 atm.
The isomerization heat of reaction is also a function of temperature. PENG-ROB predicts the heat of isomerization to vary from -7430 J/mol C4H10 at 330 K to -7080 J/mol C4H10 at 360 K.
Reactions
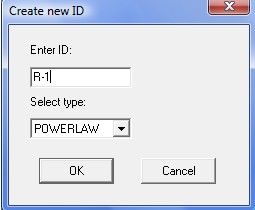
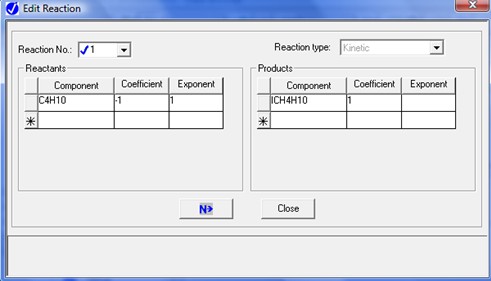
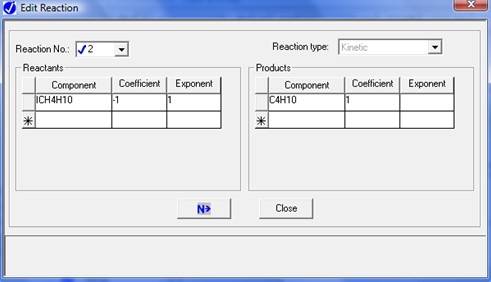
To describe the n-butane isomerization reaction, an Aspen reaction model of POWERLAW type is created: ISOMER. The ISOMER reaction is rate-controlled. The reaction stoichiometry is shown below:
![]()
Both forward and reverse reactions are 1st order with respect to reactants.
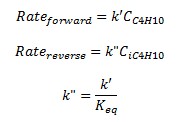
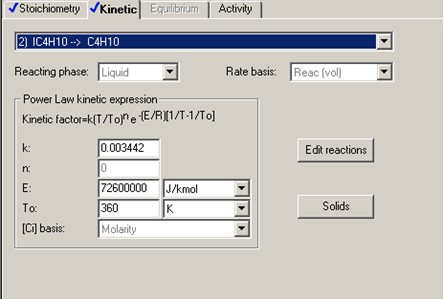
The forward reaction specific rate is 31.1 h-1 (0.008639 sec-1) at 360 K with activation energy of 65.7 kJ/mol (65.7x106 J/kmol). The reactant concentration is given in terms of molarity (kmol/m3).
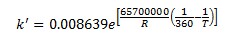
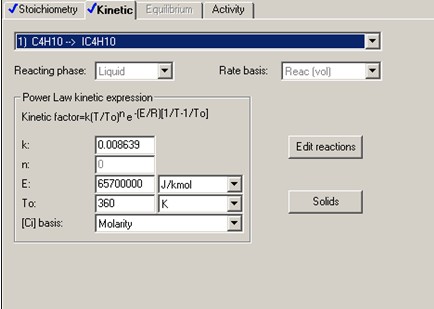
It is also known that the heat of reaction is -6900 J/mol of n-butane and the chemical equilibrium constant is 3.03 at 60°C.
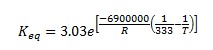
The above equation gives Keq=2.51 at 360 K. Therefore, the above equation can be rewritten as follows.
![]()
From k' and Keq, we can derive at the rate constant for the reverse reaction, k".
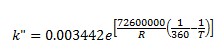
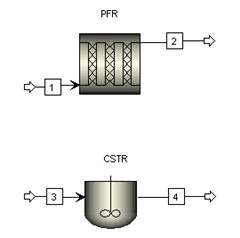
Flowsheet
Aspen model library provides RPLUG (PFR) reactor model and RCSTR (CSTR) reactor model. They are used to construct processes with proper feed streams and reactor conditions.
Problem (a)
Aspen RPLUG reactor model is used with reactor type Adiabatic Reactor and reaction model ISOMER. Aspen requires input of reactor dimensions in lieu of reactor volume. To start the simulation, initial values of 0.1 meter in diameter and 1000 meter in length are assumed.
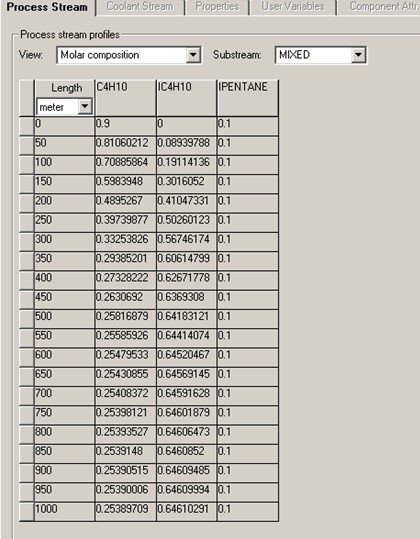
It is found that the conversion of C4H10 reaches a maximum of about 72%. 70% of C4H10 conversion is achieved with reactor length of 410 meter, or 603 second of residence time. That gives reactor volume of 3.22 m3.
Problem (b)
Profiles for the molar compositions of C4H10 and IC4H10 and the PFR reactor temperature are given below.
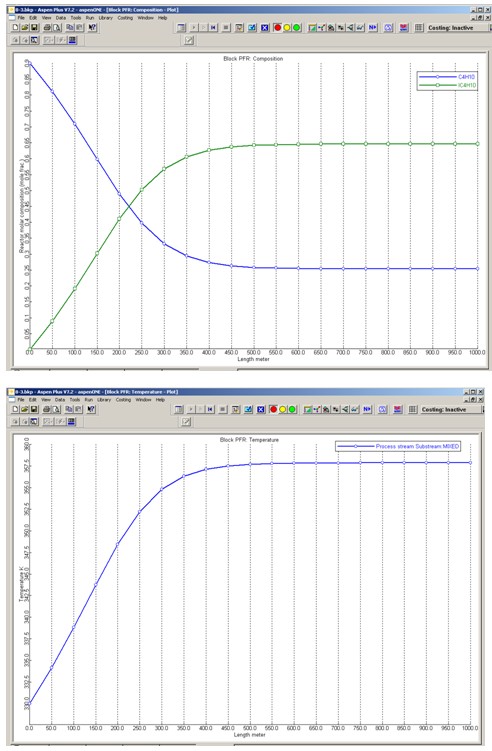
Problem (c)
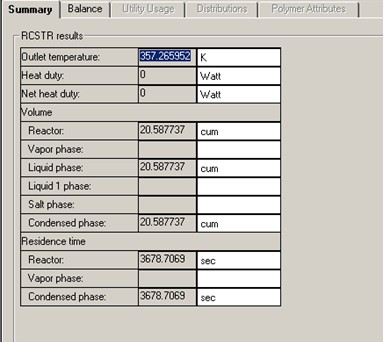
Aspen RCSTR reactor model is used with heat duty set to 0 (i.e., adiabatic) and reaction model ISOMER. To start the simulation, initial value of 3 m3 is assumed for the CSTR reactor volume. C4H10 content drops from 90 mol % to 33.7 mol % (62.5% conversion) with this initial volume of 3 m3.
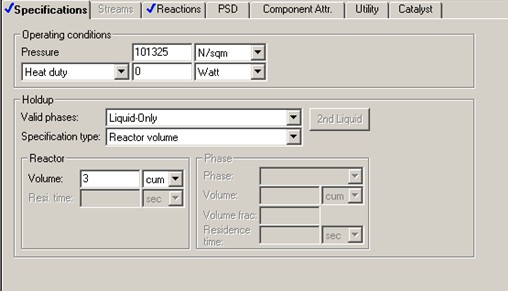
A DESIGN-SPEC block is set up to find the reactor volume required for 70% C4H10 conversion. The study shows a reactor liquid volume of 20.6 m3 is required to achieve 70% C4H10 conversion. That corresponds to a residence time of to 3678 seconds, more than 6 times that of the RPLUG residence time.
Given the models, various reactor analyses can be performed. For example, a PFR reactor liquid volume of 1.41 m3 is required to achieved 40% C4H10 conversion while a CSTR reactor requires only liquid volume of 1.30 m3.