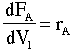
A mole balance on the moles of aluminum in the reactor is
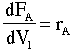 |
(1) |
Multiply both sides by Avogadro's number
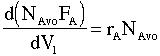 |
(2) |
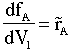 |
(3) |
where fA is the molecular
flow rate, (molecules/s), and
![]() is
the rate of formation of aluminum (molecules/s/dm3).
is
the rate of formation of aluminum (molecules/s/dm3).
Base everything on per kg of gas
|
|
(4) |
where ![]() is
the mass flow rate of gas, kg/s, and nm is the
molecular concentration of aluminum molecules per kg of gas.
is
the mass flow rate of gas, kg/s, and nm is the
molecular concentration of aluminum molecules per kg of gas.
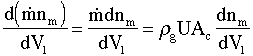 |
(5) |
If one were to generalize, one could think of the aluminum molecules as monomer molecules, in which nm is the concentration of monomer in terms of monomer molecules per kg of gas. Writing the reactor volume, V1, in terms of cross sectional area Ac and distance x down the reactor we have
|
|
(6) |
Combining Eqns (5) and (6) we obtain
|
|
(7) |
If we move with the gas,
![]() , then
, then
|
|
|
|
Dividing by rg, kg of gas/m3
|
|
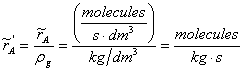 |
|
|
(8) |