2B. Growth Kinetics (Condensation Kinetics)
The newly formed nuclei can grow in size as a result of monomers hitting the particles and sticking, causing the particles to increase in size.

From the kinetic theory of gases we know the net rate of addition of the gas molecules to the particles is
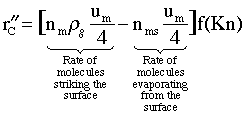
![]() (13)
(13)
The 4 in the denominator of um in Eqn.(13) is a result of averaging the molecular velocities hitting the surface from all directions. [See (Moore, W. Physical Chemistry, 2nd Ed. p167-168) to obtain the rate of molecules striking the surface per unit area.] Where
![]() = (Net number of molecules striking and sticking)/(m2
of particle)/s
= (Net number of molecules striking and sticking)/(m2
of particle)/s
![]() cm/s= gas phase velocity of molecules
cm/s= gas phase velocity of molecules
Because the gas molecules are in transition range between having small mean free paths (continuum) and large mean free paths, we need a correction factor.
[Knudsen Flow]
f(Kn) = a correction for the transition from the free molecule to the continuum regime.
[Warren and Seinfeld (1984)]
Kn is the Knudsen number ![]()
λ is the mean free path of the gas and dp is the particle diameter
The condensation parameters for the correction factor
The mean free path is given by
![]() , m (Phy. Chem, Atkins page 653, eq. 26.2.6)
, m (Phy. Chem, Atkins page 653, eq. 26.2.6)
[1.8x10-7 < λ < 3.9x10-7 m]
where ![]() collision cross-section of the gas
collision cross-section of the gas
dcg is the molecular diameter of the carrier gas (m)
dcg = 3.84x10-10 m
P is the total pressure
k is Boltzmann's constant
The rate of condensation per unit surface area is written as
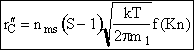 , [(# of molecules)/m2 of particle/s] (15)
, [(# of molecules)/m2 of particle/s] (15)
[0 < ![]() < 1.27x1024 molecules/m2 of particle/s for Al and the conditions given]
< 1.27x1024 molecules/m2 of particle/s for Al and the conditions given]
Summary
![]()
where N is the number of particles per kg gas and dp is the particle diameter, the net rate of loss of monomer molecules
![]()