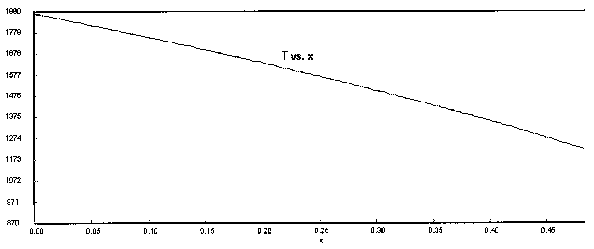
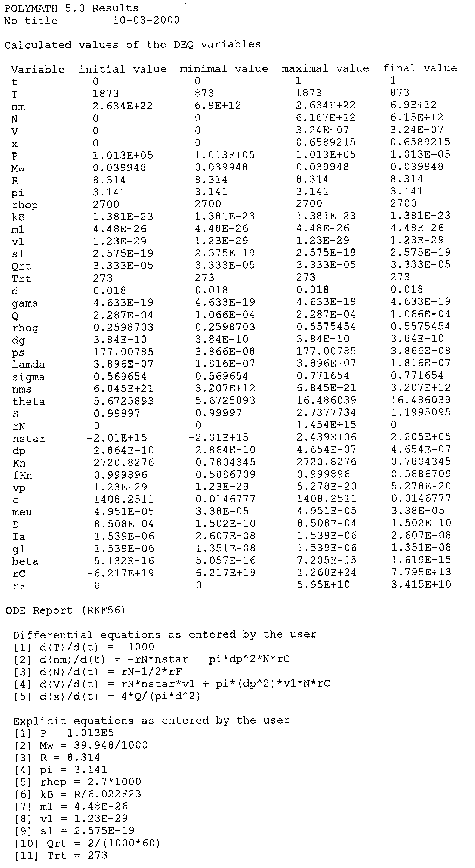
The following Polymath output graphs show the profiles for the system variables of reactor temperture, T, supersaturation ratio, S, monomer concentration, nm , particle concentration, N, nucleation rate rN, and particle diameter. Note how steep the rate of nucleation, rN, is. This gives you a feel of how stiff the differential equations describing the aerosol reactor are.
a. Effect of flow rate: No effect observed on the particle diameter.
b. Effect of Cooling Rate: Particle size decreases with an increase in the cooling rate.
c. Effect of Pressure: Particle size increases with an increase in pressure.

Figure 5. Temperature Profile
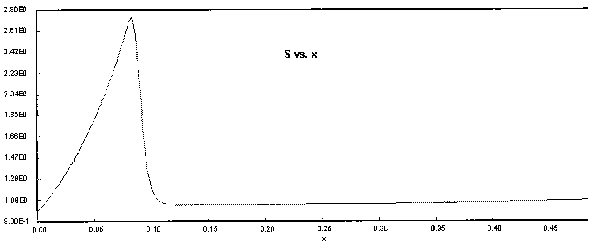
Figure 6. Super Saturation Profile
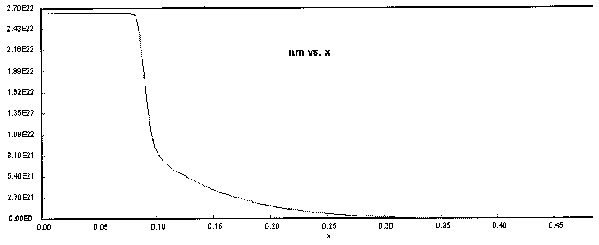
Figure 7. Monomer Concentration Profile
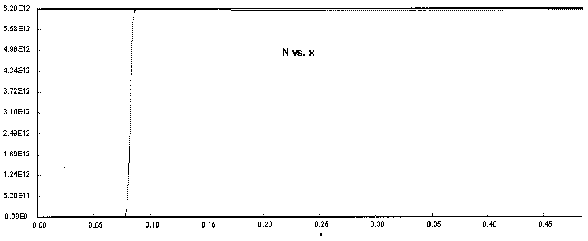
Figure 8. Particle Concentration Profile
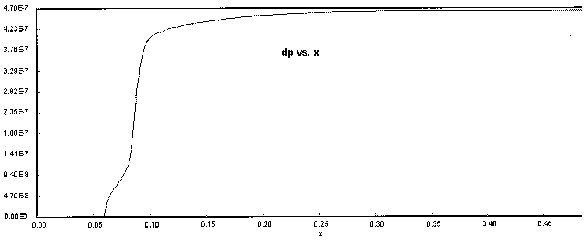
Figure 9. Particle Size Profile
nm = aluminum concentration per unit mass of gas (molecules/kg of gas)
[6.9´1012 < nm < 2.634´1022]
nms = aluminum saturation concentration (molecules/m3)
[3.2´1012 < nms < 6.8´1021]
S = super saturation Ratio = ![]() [0.999 < s < 2.74]
[0.999 < s < 2.74]
N = number of Al particles per kg of gas [0 < N < 6.1´1012]
![]() = net rate of formation (loss) of Al molecules resulting from particle nucleation and from
particle growth (condensation) (molecules/kg gas•s)
= net rate of formation (loss) of Al molecules resulting from particle nucleation and from
particle growth (condensation) (molecules/kg gas•s)
![]() = rate of nucleation of particles (no. of nuclei formed/kg of gas/s)
= rate of nucleation of particles (no. of nuclei formed/kg of gas/s)
[0 < ![]() < 1.45´1015]
< 1.45´1015]
dp = diameter of particle (m)
V1 = volume reactor (m3)
Ac = cross sectional area of reactor (m2)
![]() = carrier gas density (kg/m3) [0.26 <
rg < 0.56]
= carrier gas density (kg/m3) [0.26 <
rg < 0.56]
q is the dimensionless surface tension.
![]() [5.7 < q < 16.5]
[5.7 < q < 16.5]
s is the surface tension (Rhee, 1970), ![]() kg/s2[0.57 <
s < 0.77]
kg/s2[0.57 <
s < 0.77]
v1, s1, m1 are the volume, surface area and mass of an Al molecule respectively.
![]() ,
, ![]() and
and ![]()
ps is the vapor pressure and P is the total pressure (Pa) and temperature T.
where ![]() (22)
(22)
![]() (molecules/m3(gas)) [3.2´1012 < nms < 6.8´1021]
(molecules/m3(gas)) [3.2´1012 < nms < 6.8´1021]
![]() = rate of condensation on particles (i.e. rate of molecules hitting particle surface and
sticking) = (no. of molecules/m2 of particles/s)
= rate of condensation on particles (i.e. rate of molecules hitting particle surface and
sticking) = (no. of molecules/m2 of particles/s)
[0 < ![]() < 1.27´1024]
< 1.27´1024]
![]() = rate of flocculation of particles = (no. of particle collisions/kg of gas/s)
= rate of flocculation of particles = (no. of particle collisions/kg of gas/s)
[0 < ![]() < 5.9´1010]
< 5.9´1010]
n* = no. of molecules per nuclei (Note in development of nucleation rate g* was used but g*=n*)