
Now looking at the graph, we see the oscillations in the concentrations of A and B. Notice that the oscillations are periodic, and look like they can be modeled by sine and cosine functions. Also notice that the decline in the concentration of A, corresponding to the rise in the concentration of B appears to occur rather fast. Taking a closer look...
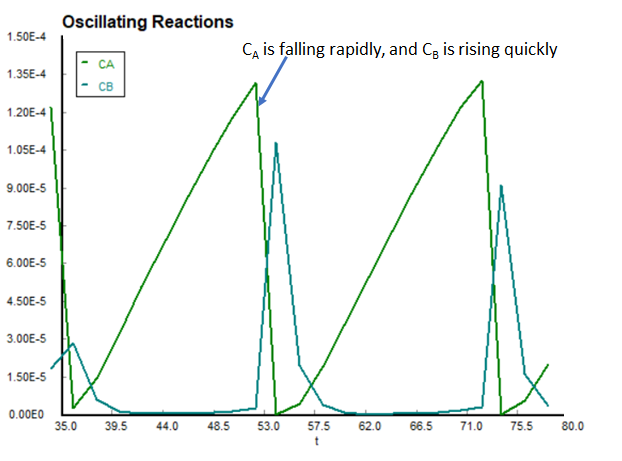
Remember again that this series of reactions is merely a simplified model to help you understand how the BZ reaction scheme works. The actual BZ scheme involves over 40 seperate reactions, and is therefore quite difficult to model. However, this does provide a good basis for the BZ reactions and their application to multiple reactions in chemical reaction engineering. With all this in mind, try to answer the following questions concerning the reaction:
1) What factors influence the amplitude and frequency of the reaction?
2) It is known that the BZ oscillations eventually cease (in the original experiment by Belousov, they lasted about 50 minutes). Why do you think that is?
3) What causes these oscillations? (In other words: What makes this reaction different than others we have studied so far?)
4) Play around with the Polymath program above- what are the effects of changing the values of k0, ku, k1, and k2? Can you make the oscillations damped or unstable?