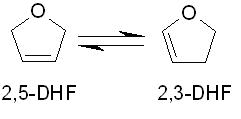
a. Determine the value of
for this reaction at room temperature and atmospheric pressure.
b. Determine the value of
at the same conditions.
c. If a container is initially loaded with pure 2,5-DHF at 298 K and 1 atm, what will be the final (equilibrium) mole fraction of 2,5-DHF?
d. If the container's temperature is subsequently increased to 600 K, what will be the final (equilibrium) mole fraction of 2,5-DHF? Assume that ideal gas conditions apply.
4.) Consider the following reaction, which occurs through a classical SN2 mechanism: