Chapter 2: Conversion and Reactor Sizing
Example
Using the Ideal Gas Law to Calculate CA0 and FA0
The entering molar flow rate for a gas is
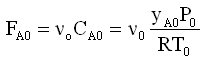
where CA0= |
entering concentration, mol/dm3 |
yA0= |
entering mole fraction of A |
P0= |
entering total pressure, e.g., kPa |
PA0= |
yA0P0 = entering partial pressure of A, e.g., kPa |
T0= |
entering temperature, K |
v0= |
volumetric flow rate |
R= |
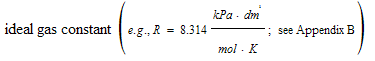 |
The size of the reactor will depend on the flow rate, reaction kinetics, reactor conditions, and desired conversion. Let's first calculate the entering molar flow rate.
A gas of pure A at 830 kPa (8.2 atm) enters a reactor with a volumetric flow rate, v0, of 2 dm3/s at 500 K. Calculate the entering concentration of A, CA0, and the entering molar flow rate, FA0.