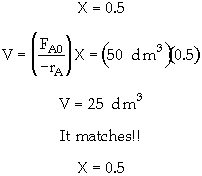Chapter 2: Conversion and Reactor Sizing
Self Test - What is Wrong with This Solution?
Problem
An adiabatic liquid phase exothermic reaction is to be carried out in a 25 dm3 CSTR. The entering molar flow rate of A times the reciprocal of the rate of reaction is shown below as a function of conversion.
What is the conversion exiting the CSTR?
Solution
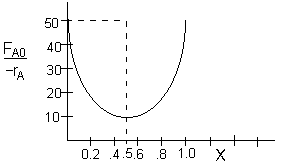
Let's calculate the area in the rectangle with the conversion at the minimum and see if it matches the volume of 25 dm3 given in the problem statement at the minimum
What is wrong with this solution?
Solution
The correct solution is trial and error using the (FAo/-rA) versus X curve. The above solution, i.e. X=0.5, is silliness as a value on the (FAo/-rA) versus X curve is never used. Guess an exit conversion, multiply by the appropriate value of FA0/-rA obtained from the graph. See if it turns out to be 25 dm3. After a few trials you will find the correct value for X, as given below.
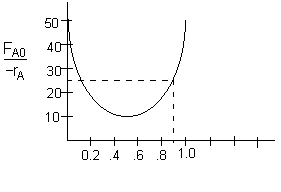
Choose X=0.885, and find (FAo/-rA) at this value.The value of
is 28.2 dm3