Chapter 5: Isothermal Reactor Design: Conversion
Chapter 5 Self Test
Zero-order Reaction in a CSTR and PFR
The zero order reaction
![]()
is carried out in a CSTR and in a PFR. For an entering volumetric flowrate of 10 dm3/s at a concentration of .5 mol/dm3, what is the space time for each reactor to achieve 90% conversion.
Additional Information: kA = 0.01 mol/dm3•s
Hint 2: CSTR combined mole balance and rate law
Hint 1
MOLE BALANCE: |
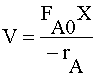 |
Hint 2
RATE LAW: |
|
COMBINE: |
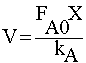 |
The zero order reaction
![]()
is carried out in a CSTR and in a PFR. For an entering volumetric flowrate of 10 dm3/s at a concentration of .5 mol/dm3, what is the space time for each reactor to achieve 90% conversion.
Full Solution: CSTR
| MOLE BALANCE: | 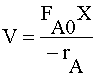 |
| RATE LAW: | |
| STOICHIOMETRY: |
|
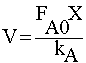 |
|
| COMBINE: | |
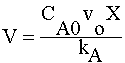 |
|
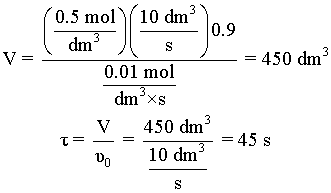 |
The zero order reaction
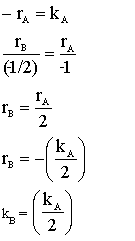
is carried out in a CSTR and in a PFR. For an entering volumetric flowrate of 10 dm3/s at a concentration of .5 mol/dm3, what is the space time for each reactor to achieve 90% conversion.
Full Solution: PFR
| MOLE BALANCE: |
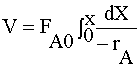 |
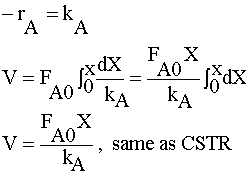
|