Chapter 5: Isothermal Reactor Design: Conversion
Self Test - Pressure Drop in a Packed Bed Reactor
The elementary isomerization
A --> B
is carried out at 20 atm in a fluidized CSTR containing 100 kg of catalyst where 50% conversion is achieved
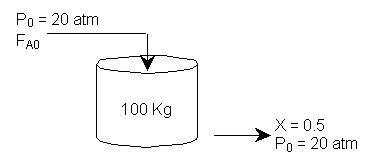
It is proposed to replace the CSTR with a packed bed reactor
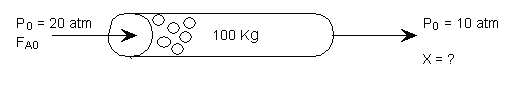
The entering pressure was 20 atm and the exit pressure was found to be 10 atm.
- What would be the conversion if No pressure drop? What information can you obtain from the CSTR?
- What will be the conversion in the new PBR with pressure drop?
How do you calculate the pressure drop parameter?
- Suppose you could choose another pipe diameter to hold the same amount of catalyst.
a) Would you choose larger pipe diameter?
b) Would you choose smaller pipe diameter?
Hint for A
CSTR
![]()
Solving for FA0/(kCA0)
![]() kg
kg
Solution for A
PFR
![]()
For no pressure drop P=P0 and y = 1
![]()
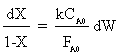
Integrating with W = 0: X=0
![]()

![]()
Hint for B
d = 0
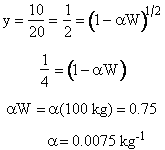
Solution for B
M.B. 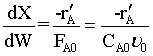
R.L. ![]()
Stoichiometry 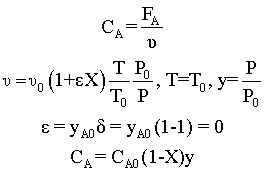
Combine 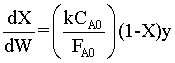
For ε = 0; y = (1-αW)1/2
Seperating Variables 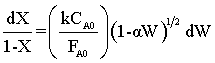
Integrating
![]()
![]()
we note
![]()
![]()
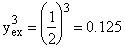
![]()

Compared with ΔP = 0 where X = 0.632
Back to problem statement
Solution for C
A plot of conversion vs. particle diameter is as follows
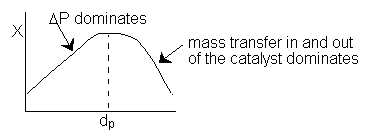
c) As the particle diameter decreases, the pressure drop increases, so the concentration decreases as does the reaction rate. Consequently when the reaction rate is small the conversion will be small. As the particle size increases the pressure drop decreases and conversion increases. As the particle size increases the pressure drop decreases and the concentration increases. however, for large particles, it takes a long time for the reactants to diffuse in and out of the catalyst particle. (I.e. -rA ∼ 1/dp therefore k ∼ 1/dp, see p.829, last equation on the page where R = dp/2 .)
Solution for D
d) You would like to have the biggest pipe diameter possible because the bigger the pipe diameter, the less the pressure drop, and thus the conversion.