Chapter 8: Multiple Reactions
Additional Homework Problems
CD8-15B
The hydrogenation of benzene (B) is carried out in a CSTR slurry reactor where the desired product is cyclohexene (C) [Chem. Eng. Sci., 51, 2873 (1996)].
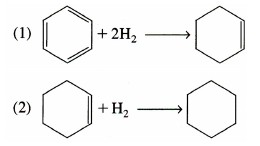
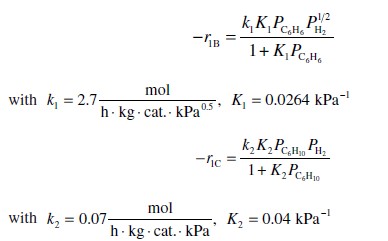
The rate laws for reactions (1) and (2) at 403 K are
The rate laws are valid over the following range of partial pressures
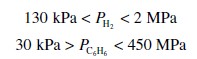
The entering molar flow rate of benzene is 2 mol/s and it is necessary to achieve at least 40% conversion of benzene at 403 K.
(a) At what entering conditions should the reaction be carried out to maximize the yield of cyclohexene?
(b) For part (a), plot the molar flow rates as a function of PFR volume.