Chapter 8: Multiple Reactions
Additional Homework Problems
CDP8-26B
The following hydrodealkylation reactions occur over a Houdry Detol catalyst near 800 K and 3500 kPa:
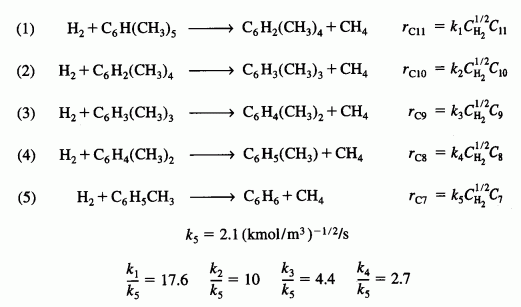
The feed is equimolar in hydrogen and pentamethylbenzene.
- For an entering volumetric flow rate of 1 dm3/s, what ratio of hydrogen to pentamethylbenzene and what PFR reactor volume would you recommend to maximize the formation of C6H4(CH3)2 (i.e., C8)? [Hint:Plot the overall selectivity as a function of reactor volume.]
- How would your answer change if you were to maximize the overall selectivity of C8 to C9, i.e.S89? Of C8 to C7?
- What do you think the point of this problem to be?
Make a plot of the mole fraction of each component as a function of conversion of pentamethylbenzene. Make a plot of the mole fraction of each component as a function of PFR volume. Discuss any optimization that could be done.
[3rd Ed. P6-16]