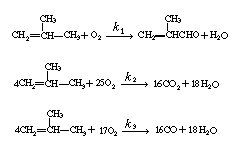Chapter 8: Multiple Reactions
Additional Homework Problems
CDP8-CB
| Isobutylene is catalytically oxidized to methacrolein, CO 2 , and CO. Data, in terms of three pseudo-first-order, parallel, competing reactions, follow: | ||||||||||
|
|
||||||||||
|
||||||||||
| The reactor is a fluidized bed of catalyst particles. Use a CSTR model to calculate the expected conversions of i -butylene for all reactions under the following conditions: | ||||||||||
|
|
||||||||||
| (O. M. Fuller, McGill University) [1st Ed. P9-16] |
||||||||||