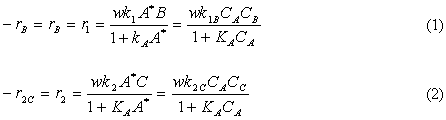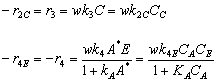Chapter 8: Multiple Reactions
CDP8-GC
A new catalytic pathway for an important intermediate in the production of nonsteroidal anti-inflammatory drug ibuprofen (e.g., Advil) has been developed [Chem. Eng. Sci., 51, 10, 1663 (1996)]. The pathway involveds the hydrogenation of p-isobutyl acetophenone (B) in a solution of methanol containing a HY seolite catalyst and saturated with hydrogen. The intermediate products are p-isobutylethyl ithanol (C), p-isobutylphenylethylmethyl ether (E) and p-isobutylphenyl benzene (F). The reaction scheme is shown below. The following rate laws apply to the above equation.
(1) A + B
C
(2) C + A
F + H2O
(3) C + M
E + H2O
(4) E + A
F + M
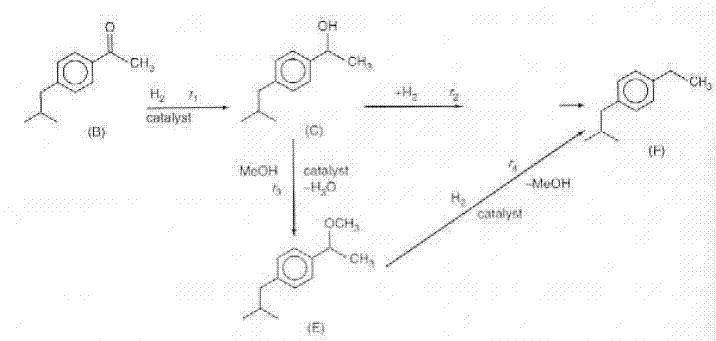
Because methanol, M, is in excess, it does not appear in the rate law. The rate and equilibrium concstants are given in Table P6-26. the catalyst charge, w, iis 10 kg/m3 and the initial concentration of p-isobutyl acetephenone, (B) is 0.54 kmol/dm3, the partial pressure of hydrogen is 5.9 MPa, and the temperature of 393 K. Plot the concentrations of A, B, C, D, E, and F as a function of time.
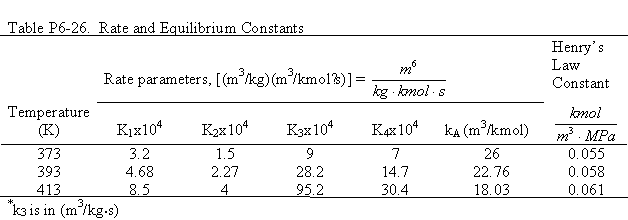
[3rd Ed. P6-26]