Chapter 8: Multiple Reactions
Finding the Selectivity
For the elementary reactions:
![]()
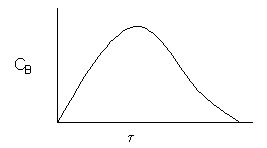
with k1=.1 s-1 and k2=.2 s-1 with CAO= 2 mol/dm3. Plot the concentration of B and selectivity of B to C as a function of space time in a CSTR.
|
|
For the elementary reactions:
![]()
with k1=.1 s-1 and k2=.2 s-1 with CAO= 2 mol/dm3. Plot the concentration of B and selectivity of B to C as a function of space time in a CSTR.
Solution
![]()
CSTR
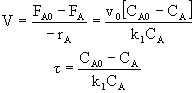
![]()
B.Balance on Species B
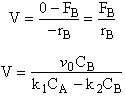
![]()
C.Balance on Species C
![]()
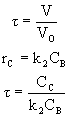
![]()
![]()
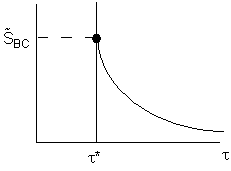
The space time,![]() , is the minimum value of V (i.e.
, is the minimum value of V (i.e.![]() ) at which there is an acceptable molar flow rate of the desired product B from the CSTR.
) at which there is an acceptable molar flow rate of the desired product B from the CSTR.
![]()