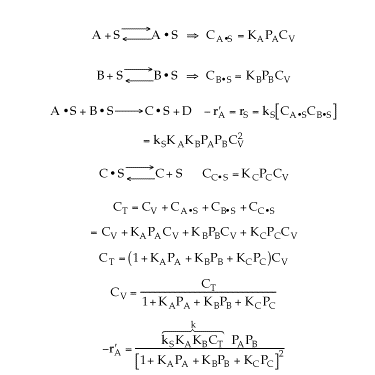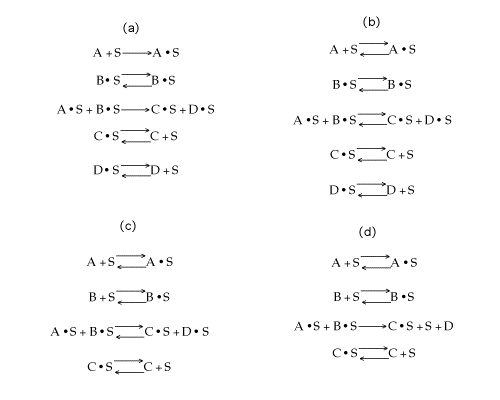Chapter 10: Catalysis and Catalytic Reactors
Finding the rate law and mechanism for A+B<=>C+D
The following data were reported for the reaction A + B goes to C + D
Which of the following mechanisms is consistent with the above data?
Solution
Answer:(d)
Recall
-r'A=-r'B=r'C=r'D
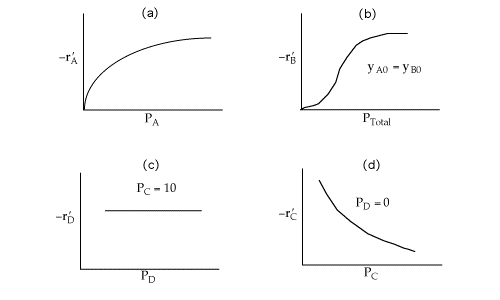
Figure (a) suggests
![]()
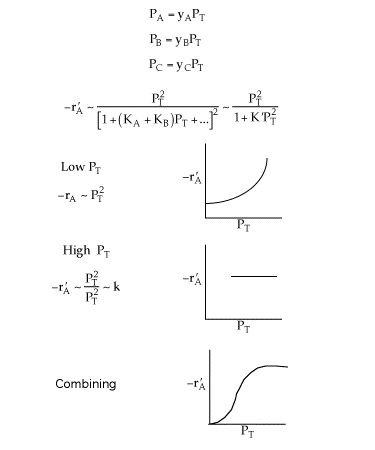
Figure (b) suggests
![]()
Figure (c) suggests
-rA is not a function of PD, therefore the reaction is irreversible and D is not on the surface.Figure (d) suggests
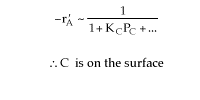
Combining all the above
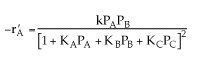
Therefore, (d)is consistent