Chapter 11: Nonisothermal Reactor Design: The Steady State Energy Balance and Adiabatic PFR Applications
Frequently Asked Questions (FAQs)
- Can XEB for a non-adiabatic reactor be linear? Under what
conditions?
Yes. Most all cases because deltaCP (T-Ta) is small wrt the heat of reaction.
- When doing an energy balance on a PBR, should we ever take into account
the properties of the catalyst? (i.e. CP of the catalyst)
Only for moving bed reactors and fluidized CSTR's, in which the catalyst is fed to the reactor along with the reactants, operated at steady state and for other reactors operated at unsteady state.
- Will we do any "semibatch" reactor problems where the entering flowrate
changes? Would this be helpful toward the end of the reaction?
Sometimes. Maybe, depends if unwanted side reactions are present.
- Why doesn't the stirring involved in a CSTR count towards shaft
work (WS)?
It does count. It will be a negative value (e.g. -10,000 cal/s) because work is being done on the system. A viscous liquid would increase in temperature with rapid stirring.
- What are the effects of inerts on
 ?
?
The inerts do not affect
 . Delta Cp only involves the reating species. For example
. Delta Cp only involves the reating species. For example

- What are the effects of inerts?
Inerts will decrease the temperature reise for an exothermic reaction and decrease the temperature drop for an endothermic reaction.
- Do you include products and inerts in
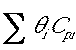 ?
?
Only include those species which are entering the reactor. Remember
