Chapter 12: Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Chapter 12 Example
Finding when R(T) = G(T)
Part 1
For an entering temperature of 340 K and an ambient temperature of 310, find the reactor temperature and conversion.
Part 2
Find X and T when the flow rate is increased by a factor of 4.
The following curve gives the G(T) for the reaction.
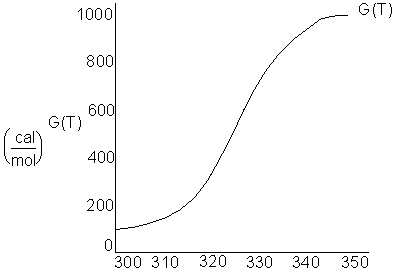
The plateau region of the curve gives the heat of reaction via the following pathway.
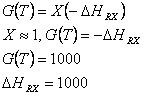
Solution
Part 1
![]()
The heat removal curve is
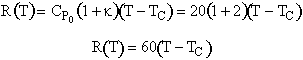
The intercept T = TC is
This line is shown on the figure below. We see that ![]() K
K
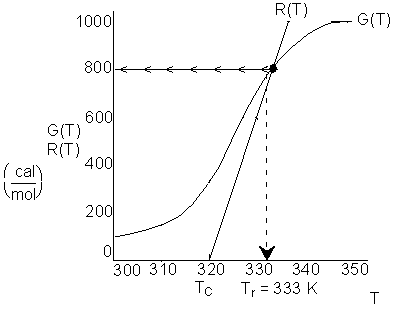
From the intersection of R(T) and G(T), we find that the steady state reactor temperature is 333 K. To find the conversion:
G(T) = X(- DHRx)
@ Intersection of G(T) and R(T), G(T) = R(T) = 800
![]()
The conversion is 80%
Part 2
We now consider another situation where the flow rate is increased by a factor of 4 and the inlet temperature is 330K and the ambient temperature is 300K.
Solution
![]()
For case 2 we find that
The heat removal line is now
and the intercept is
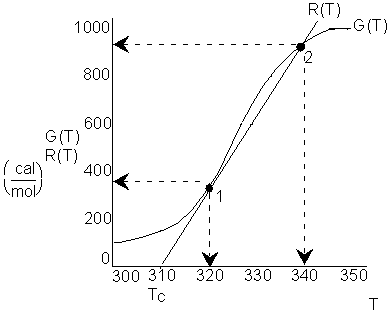
We see that ![]() at two points, consequently there are two possible
steady states for the temperatures inside the reactor, one at 320K and
one at 340K. The corresponding conversions are
at two points, consequently there are two possible
steady states for the temperatures inside the reactor, one at 320K and
one at 340K. The corresponding conversions are
and
![]()