Chapter 12: Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Example - Heat Exchange with Constant Ta
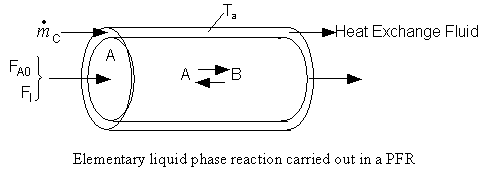
The feed consists of both inerts I and Species A with the ratio of inerts to the species A being 2 to 1.
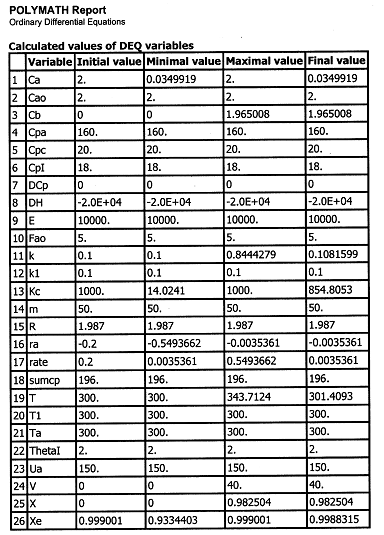
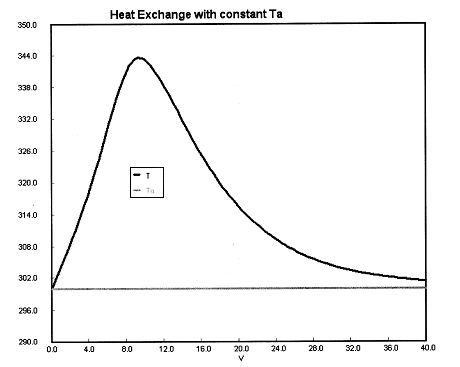
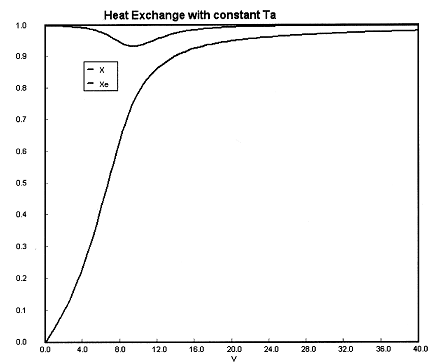
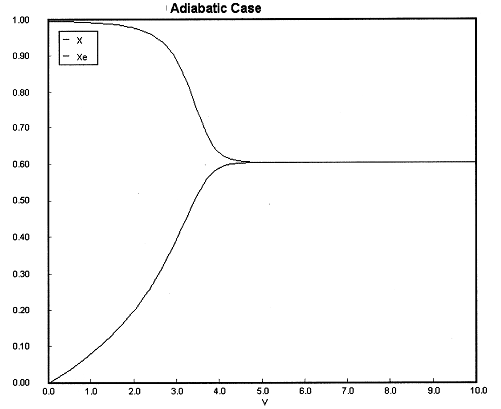
(a) Constant Ta. Plot X, Xe, T, Ta and Rate of disappearance of A when there is a heat loss to the coolant and the coolant temperature is constant at 300 K for V = 40 dm3. How do these curves differ from the adiabatic case?
Reactor

| Mole Balance | ||
| (1) | ||
| Rate Law | ||
| (2) | 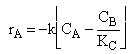 |
|
| (3) | 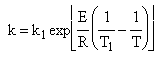 |
|
| (4) | 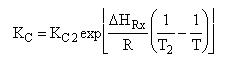 |
|
| Stoichiometry | ||
| (5) | ||
| (6) | ||
| Parameters | ||
| (7)-(15) | ||
| Energy Balance |
||

(a) Constant Ta.