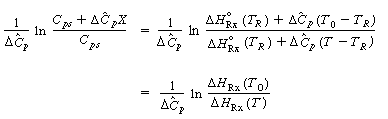Chapter 13: Unsteady State Nonisothermal Reactor Design
Professional Reference Shelf
R13.6 Adiabatic Operation of Batch Reactors
| For adiabatic operation |
|||
|
|
(13-13) | ||
| where |
|||
|
|
(13-15) | ||
| multiplying by dt, separating variables and integrating between the initial conditions, X = 0 and T =T0, and later conditions X =X and T =T, yields | |||
|
|
|||
| Taking the antilog, we have | |||
|
|
|||
| solving for X yields | |||
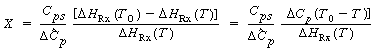 |
|||
| Then | |||
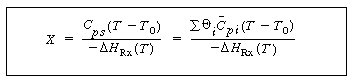 |
(13-16) | ||
| This form is the same as the equation relating X and T derived for flow reactors. Rearranging yields | |||
|
Temperature |
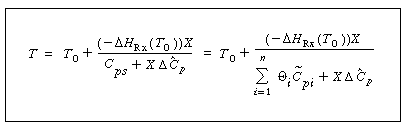
|
(13-17) |