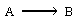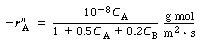Chapter 14: External Diffusion Effects on Heteregeneous Reactions
Additional Homework Problems
CDP14-AB
| The reaction | ||
|
||
| is carried out on a metallic surface and follows Langmuir-Hinshelwood kinetics at room temperature with the corresponding rate law: | ||
|
||
| However, at higher temperatures the reaction is mass transfer-limited. The reaction currently takes place on a monolith catalyst where 45% conversion is achieved. It is proposed to double the number of plates for the same width and to halve the length of the reactor. What conversion can be expected for
[2nd Ed. P10-11] |