Chapter 15: Diffusion and Reaction in Porous Catalysts
Additional Homework Problems
CDP15-CB
The catalytic hydrogenation of methyl linoleate to methyl oleate was carried out in a laboratory-scale slurry reactor in which hydrogen gas was bubbled up through the liquid containing spherical catalyst pellets. The pellet density is 2 g/cm3. The following experiments were carried out at 25°C:
| Run | Partial Pressure of H2 (atm) |
Solubility of H2 (g mol/dm3) |
H2 Rate of Reaction (g mol/dm3  min) min) |
Catalyst Charge (g/dm3) |
Catalyst Particle Size ( 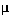 m) m) |
| 1 | 3 | 0.007 | 0.014 | 3.0 | 12 |
| 2 | 18 | 0.042 | 0.014 | 0.5 | 50 |
| 3 | 3 | 0.007 | 0.007 | 1.5 | 50 |
- It has been suggested that the overall reaction rate can be enhanced by increasing the agitation, decreasing the particle size, and installing a more efficient sparger. With which, if any, of these recommendations do you agree? Are there other ways that the overall rate of reaction might be increased? Support your decisions with calculations.
- Is it possible to determine the effectiveness factor from the data above? If so, what is it?
- For economical reasons concerning the entrainment of the small solid catalyst particles in the liquid, it is proposed to use particles an order of magnitude larger. The following data were obtained from these particles at 25°C:
The Thiele modulus is 9.0 for the 750-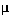 m particle size in run 4. Determine (if possible) the external mass transfer coefficient, kc, and the percent (of the overall) of the external mass transfer resistance to the catalyst pellet.
m particle size in run 4. Determine (if possible) the external mass transfer coefficient, kc, and the percent (of the overall) of the external mass transfer resistance to the catalyst pellet.
| Run | Partial Pressure of H2 (atm) |
Solubility of H2 (g mol/dm3) |
H2 Rate of Reaction (g mol/dm3  min) min) |
Catalyst Charge (g/dm3) |
Catalyst Particle Size ( 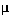 m) m) |
| 4 | 3 | 0.007 | 0.00233 | 2.0 | 750 |
[3rd Ed. P12-20]