Chapter 17: Distributions of Residence Times for Chemical Reactors
Additional Homework Problems
WEB-P17B-A
An isothermal pulse test on a piece of reaction equipment gave the following results: The output concentrations rose linearly from zero to 0.5 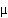 mol/dm3in 5 min, then fell linearly to zero in 10 min ager reaching the maximum value.
mol/dm3in 5 min, then fell linearly to zero in 10 min ager reaching the maximum value.
(a) Calculate in tablular form the values of E(t) and F(t) at 1-min intervals. Sketch these functions.
(b) What is the mean residence time? If the flow were 150 gal/min, what would be the total reactor volume? (Ans: tm = 6.67 min, V = 1000 gal)
A second order reaction with kCA0 = 1.2 min-1 at 325 K is carried out in the system.
(c) If the reactor were plug flow with the same flow and volume, what would be the conversion? (Ans.: X = 0.889.)
(d) If the reactor were a CSTR with the same flow and volume, what would be the conversion? (Ans.: X = 0.703.)
(e) If the flow were completely segregatedwith the F(t) above, what would be the conversion? (Ans.: X = 0.86.)
(f) If the flow were in a state of maximum mixedness with the E(t) above, what should be the conversion?
(g) How would your answrs to part _____ (to be assigned) change if the reaction were carried out adiabatically with the paramenter values given by Equation P13-2(h).1]?
(h) What if the reaction were endothermic with E = 10,000 cal/mol and carried out adiabatically with
T(K) = 325 - 500X
How would your answers change?