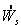 and
and 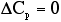 , sketch conversion as a function of temperature.
, sketch conversion as a function of temperature.
For an adiabatic reaction withSolutionand
, sketch conversion as a function of temperature.
|
|
For and adiabatic reaction withand
, sketch conversion as a function of temperature.
A. For an exothermic reaction, is negative ΔHRX(-),[ e.g., ΔHRX= -100 kJ/mole A]
X increases with increasing T.
B. For an endothermic reaction,ΔHRX is positive (+), XEB increases with decreasing T.
For a first order reaction
Both the mole and energy balances are satisfied when XMB=XEB. The steady state temperature and conversion are TSSand XSS respectively for an entering temperature TO.