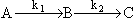
For the elementary reactions:Solutionwith k1=.1 s-1 and k2=.2 s-1 with CAO= 2 mol/dm3. Plot the concentration of B and selectivity of B to C as a function of space time in a CSTR.
|
|
For the elementary reactions:with k1=.1 s-1 and k2=.2 s-1 with CAO= 2 mol/dm3. Plot the concentration of B and selectivity of B to C as a function of space time in a CSTR.
CSTR
A. Balance on Species A
B.Balance on Species B
C.Balance on Species C
The space time,
, is the minimum value of V (i.e.
) at which there is an acceptable molar flow rate of the desired product B from the CSTR.