
 |
|
The differential equation for a PFR with catalyst is :
Assuming that the reaction is elementary since we are not told otherwise, the reaction rate is defined as :
through stoichiometry, this becomes:
Substituting the reaction rate back into the differential equation gives :
which can be integrated from X=0 when W=0 to X=X when W=W :
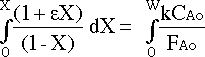
to give :
Placing the known values into the equation :
Solving gives a conversion of :
![]() Back to Heterogeneous Example 1
Back to Heterogeneous Example 1