The radius at which the heat had been raised sufficiently for the structural breakdown to take place (i.e. the radius of the iodine rings) was measured as a function of time. The ratio of cooked potato volume and the total volume was plotted with respect to time for three different oven temperatures. As one can see the data suggests that the potato actually "uncooked" after 25 minutes in the oven at a temperature of 300° F. Uncooking a potato is of course impossible. Once the crystalline structure of the potato has been broken down it can not regenerate. The dataset collected at 300° F should therefore not be considered when further conclusion regarding the reaction are drawn.
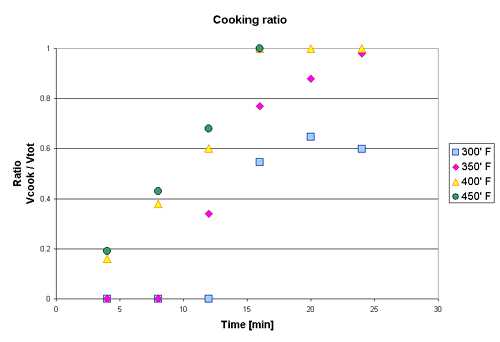
The second plot shows the trendlines attached to the data points of the three higher temperatures. When attaching the trendlines, which describe the reaction, the data points prior to the start of the reaction and the data points which represent full conversion are not used. As can be seen below the fit of a straight trendline to the data seems to increase at higher temperatures. The difference between baking a potato at 400° F and at 450° F seems to be almost neglible and raising the temperature over 450° F would probably just burn the skin of the potato while not shortening the actual baking time

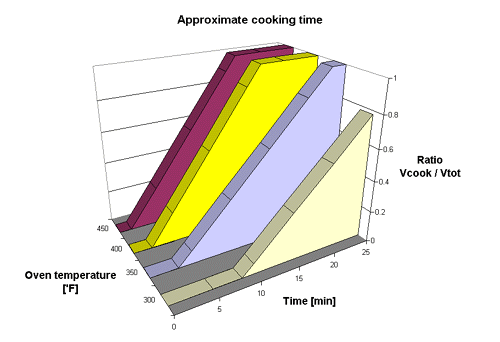
The third and last plot shows in a graphic way the approximate time it takes to break down the crystalline structure of a potato at four different temperatures. However as mentioned above the accuracy increases as the temperature increases so that while the part of the chart representing 300° is a relativly poor model of the reality the part representing 450° seems to model it quite good.