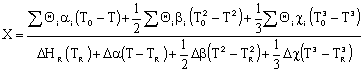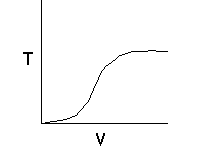
Let's calculate the volume necessary to achieve a conversion, X, in a PFR for a first-order, exothermic reaction carried out adiabatically. For an adiabatic, exothermic reaction the temperature profile might look something like this:

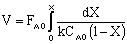
, 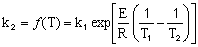



Need to relate X and T. Use the Energy Balance to

The user friendly forms of the energy balance we will focus on are outlined in the following table.
In the material that follows, we will derive the above equations.
Energy Balance: 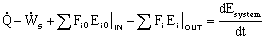
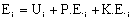
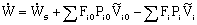
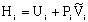

General Energy Balance: 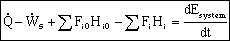

For steady state operation: 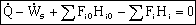


We need to put the above equation into a form that we can easily use to relate X and T in order to size reactors. To achieve this goal, we write the molar flow rates in terms of conversion and the enthalpies as a function of temperature. We now will "dissect" both Fi and Hi.
Adiabatic Energy Balance: 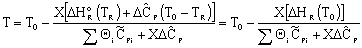
Adiabatic Energy Balance for variable heat capacities: