P4-? Membrane Reactor
The gas phase reversible isomerization
2A ![]() 2B
2B
follows an elementary rate law and is to be carried out in a membrane reactor (IMRCF). Owing to the configuration of species B, it is able to diffuse out the walls of the membrane, while A cannot. The equilibrium constant is KC = 1.0.
(A) An equal molar mixture of A and inert, I, (50% A) is fed to conventional PFR.
1) What is the equilibrium conversion?
Below is a sketch of the conversion profile for the conventional PFR.
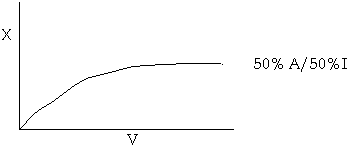
Sketch and label the profile on the same plot when the total molar flow rate remains the same but the feed composition is
2) 100% A
3) 10% A
(B) An equal molar mixture of A and I is fed to a IMRCF in which only B diffuses out of the reactor.
1) What is the equilibrium conversion?
Below is a sketch of the equilibrium conversion profile for a conventional PFR.
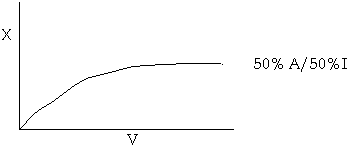
On the same plot sketch and label the IMRCF conversion profile for
2) A small coefficient of the permeability (i.e. mass transfer coefficient) kCB; and
3) A very very very large value of kCB.
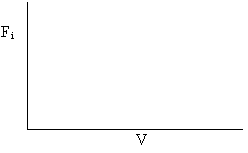
On the figure below sketch the molar flow rates FA, FB, and FI as a function of IMRCF volume.
For kCB = 0
For large kCB
(C) An equal molar mixture of A and I is fed to an IMRCF in which both B and I can diffuse through the membrane. Below is a sketch of the conversion profile for a conventional PFR
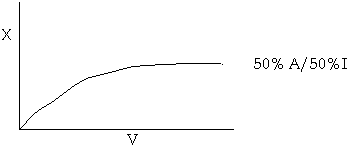
On the same plot sketch and label the conversion profile for various rates of transport of I out of the reactor, (i.e. vary kCI) but at a fixed, but moderate value of the transport coefficient of B, kCB.
1) When kCI = 0
2) When kCI is very very very large.
Sketch and label the profiles for FA, FB, and FI for the same conditions 1) and 2) above.

(D) Write the algorithm for the case when both B and I diffuse through the membrane.
(E) For Part (D) above, write the complete POLYMATH program in POLYMATH notation that will give the conversion and molar flow rate profiles down the reactor. ![]()