- How would the problem involving three reactors in series in Chapter 2, on page 61, change if there were sidestreams between?
One can't use the definition of total conversion up to a point because the reactant is fed to the stream between reactors under these circumstances.
One must work in terms of molar flow rates when writing the mole balances.
- Is there ever a time when a CSTR will have a lower volume than a PFR for the same conversion and flow rates?
Yes, for some adiabatic, exothermic reactions.
- In the 3-reactor series, (CSTR, PFR, CSTR) why wouldn't you just use the PFR to have the least volume overall to achieve the best conversion?
You would if you had a PFR large enough.
We are assuming that you have these reactors available for your use.
See the ICM-Staging.
- For the three reactors in series example, can a PFR use liquid like the 2 CSTRs?
Yes.
- If you had three reactors and two were CSTR's and the other was a PFR, would the PFR be placed at the end to minimize volume?
Yes, in most instances when the reaction is isothermal and the curve of (1/-rA) increases monotonically (i.e. no valleys or mountains) with X.
- Does
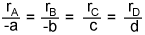 (for aA + bB
(for aA + bB
 cC + dD) only hold for first order reactions?
cC + dD) only hold for first order reactions?
No! This relationship has only to do with stoichiometry and nothing to do with rate laws.
It holds for reactions of ANY order.
- How do you use the mole balance equations to model reactors in parallel?
See Example 4-2 (p.142-146).