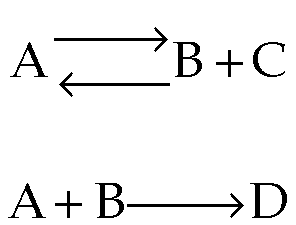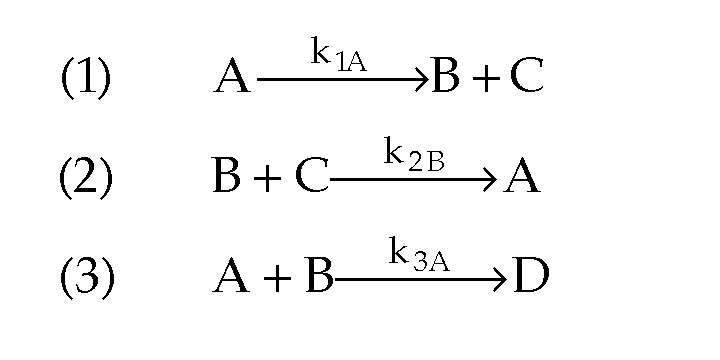- When do you need to consider overall selectivity (or yield)? What is
the advantage of having the two different kinds of selectivity (or yield)?
The instantaneous selective guides our initial reactor selection. For
a CSTR the instantaneous selectivity is the same as overall selectivity.
- What is the purpose of the instantaneous selectivity parameter? It
seems to be just a descriptive term?
No, it helps to synthesize reaction schemes (see p.285-298).
- Why do you use a CSTR if you want to keep CA low and a
PFR when you want it high? This refers specifically to the home problem?
CSTRs operate at the lowest concentration. Feed drops to the low concentration
immediately upon entry. PFR concentration starts high at entrance and drops
slowly as one moves down the reactor.
- I'm confused about space time. Is it characteristic of a reactor
(space time = overall volume/ volumetric flow rate), which I know to be
the case when the volume is fixed, but is it the case for PFR/PBR? Or,
for flow reactors, does it mark the position in the reactor just like V
does (space time = current volume in the reactor/ volumetric flow rate)?
I see plots of parameters (e.g. X, FA) vs. space time, I am
confused whether you're talking about different reactors (giving different
space times) or different positions in the same reactor. Please see
Figures E6-6.1 and E6-7.1
For a CSTR it is always the total volume divided by the volumetric flow
rate. Either the flow rate or total reactor volume may be varied to vary
t shown in Fig. E6-7.1. For a PFR space time can be related to the
location down the reactor but it also represents the total volume divided
by the volumetric flow rate where either one or the other is varied to
vary the space time t.
- What happens when you have multiple reactions that are reversible?
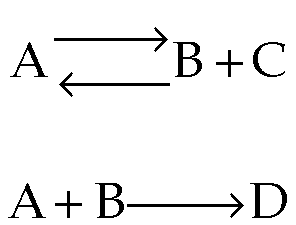
Just treat as:
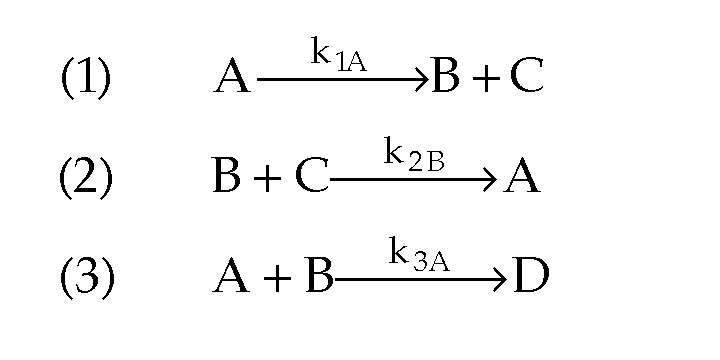
When there are parallel reactions, the book discusses many cases
comparing the reaction orders of the equations. What kind of reactor should
be used if the two reactions have the same order (a1
= a2)?
In this case you need to work with temperature dependence to affect
selectivity.
- In chapter 6, we consider the multiple reactions under isothermal
conditions, and we will cover in later chapters non-isothermal multiple
reactions. Is temperature the only main concern in the design for maximizing
selectivity?
We can vary inlet conditions and type of reactors
- Can catalysts be used in a competing reaction scenario? Can they
be used to give more of a preferred product?
Yes. Definitely!