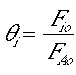Yes. Most all cases because deltaCP (T-Ta) is small wrt the heat of reaction.
Only for moving bed reactors and fluidized CSTR's, in which the catalyst is fed to the reactor along with the reactants, operated at steady state and for other reactors operated at unsteady state.
One must take ratio of the two! Usually when (UA/mCP) < 0.1, you can expand exponents in a Taylor series to get Q = UA(Ta - T).
Good point!! Yes it does.
See P13-8, p.865.
Sometimes. Maybe, depends if unwanted side reactions are present.
It does count. It will be a negative value (e.g. -10,000 cal/s) because work is being done on the system. A viscous liquid would increase in temperature with rapid stirring.
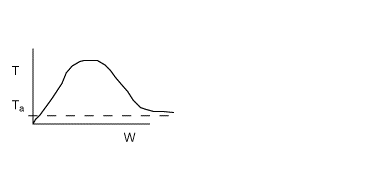
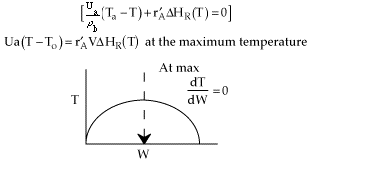
No! The term dT/dW will only equal zero if there is a maximum
in the temperature down the length (i.e. W) of the packed bed. For a single
reaction, the maximum will only occur if the reaction is exothermic and
there is heat exchange.
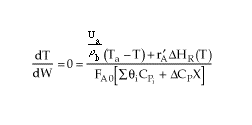
then
A lower activation energy means the reaction is less temperature sensitive. The G(t) curve also depends on t. See Fig. 8-21, p.494.
If there is no chance to add water, run for the door.
It simply shifts the R(t) curve. It is easily included in the heat removed
term
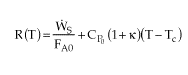
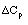 ?
?
The inerts do not affect 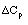 . Delta Cp only involves the reating species. For example
. Delta Cp only involves the reating species. For example
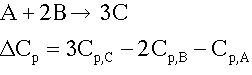
Inerts will decrease the temperature reise for an exothermic reaction and decrease the temperature drop for an endothermic reaction.
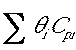 ?
?
Only include those species which are entering the reactor. Remember