| Batch Systems with Heat Effects Example | top |
Balance on a system volume that is well-mixed:
![]()
Adiabatic batch reactor with no work: ![]()
The following reaction occurs in a batch reactor:
1) |
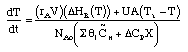 |
2) |
|
3) |
|
4) |
|
5) |
|
6) |
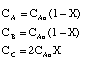 |
7) |
Parameter Values |
Adiabatic Reaction
|
|
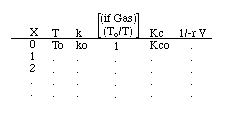 |
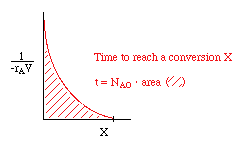 |
or use one of the integration formulas, e.g.: |
|
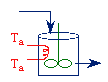
Cooling:
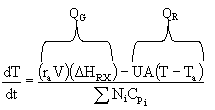
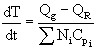
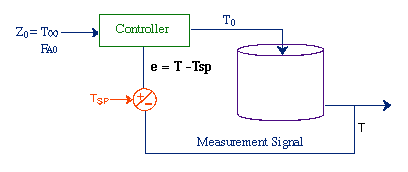
| Control of Chemical Reactors | top |
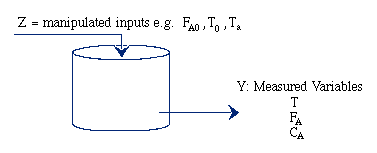 |
 |
Unsteady State CSTR: |
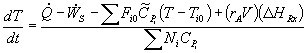 |
For a batch reactor, FAO = 0 |
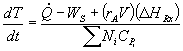 |
| For the reaction |
|
 |
|
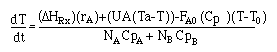 |
|
![]()
![]()
| Linearized Stability Theory | top |
| Energy Balance (Applied to a CSTR) | |
1. |
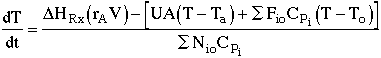 |
2. |
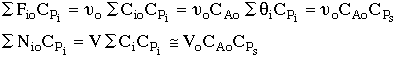 |
3. |
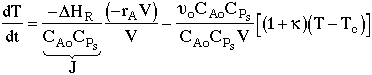 |
4. |
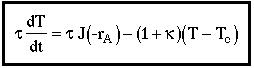 |
| CSTR Mole Balance |
|
5. |
|
6. |
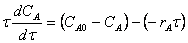 |
| Manipulating the Energy and Mole Balances |
|
Let |
|
7. |
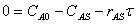 |
Adding equations (6) and (7): |
|
8. |
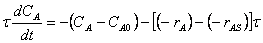 |
Linearizing |
|
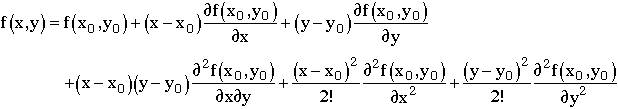 |
|
To obtain: |
|
9. |
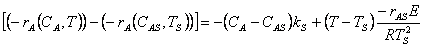 |
10. |
Let |
| |
|
| |
|
11. |
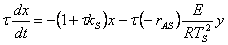 |
12. |
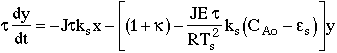 |
13. |
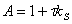 |
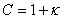 |
|
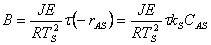 |
|
Using these substitutions, we can arrive at the following equations that describe the behavior of temperature and concentration, when the steady state conditions are perturbed in a CSTR: |
|
14. |
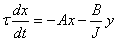 |
15. |
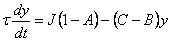 |
16. |
|
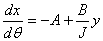 |
|
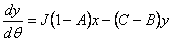 |
|
17. |
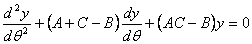 |
18. |
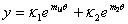 |
19. |
|
20. |
|
21. |
|
22. |
|
| Predicting the Behavior of a CSTR using LST | top |
At time t = 0, y1 = y10, where:
![]()
Making use of Equation 20
![]()
we notice that for the case of b2 = 4c:
if b < 0 , then the amplitude (i.e., T - TS) will increase
if b > 0 , then the amplitude (i.e., T - TS) will decrease
and that:
if b2 > 4c , then![]() is real (i.e., non-oscillatory behavior)
is real (i.e., non-oscillatory behavior)
if b2 < 4c , then![]() is imaginary (i.e., oscillatory behavior)
is imaginary (i.e., oscillatory behavior)
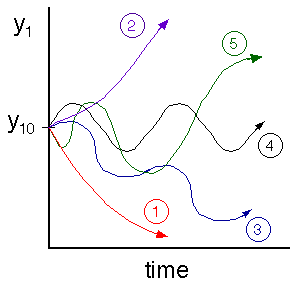 |
||
|
||
Reference: |
||
* All chapter references are for the 4th Edition of the text Elements of Chemical Reaction Engineering .