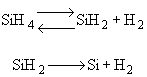
We found that
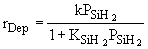
The first reaction is in equilibrium
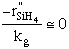
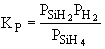
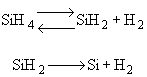 |
||
|
We found that |
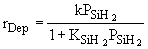 |
|
|
The first reaction is in equilibrium |
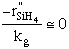
|
|
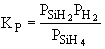 |
Case A: Small Consumption of SIH2 |
In many cases a very thin film of the material is deposited
(a few molecular layers) and as a result very little SiH2 is
consumed. For example if SiH4 dissociates to form 1 mole SiH2
and 1 mole of H2 at equilibrium and then 0.01 mole of SiH2
is consumed in the deposition, then for all practical purposes 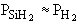 (i.e., 0.99 » 1.0). Consequently, (i.e., 0.99 » 1.0). Consequently,
|
|
|
| Case B: Significant Consumption of SIH2 |
One the other hand if significant amounts of SiH2
are consumed, we can still obtain 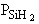 from the equilibrium relationship. However from the equilibrium relationship. However 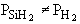 and we must include and we must include 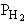 in the rate law in the rate law |
|
|
| which is the same as Eqn. below (S10-12) on p666. |
|
|