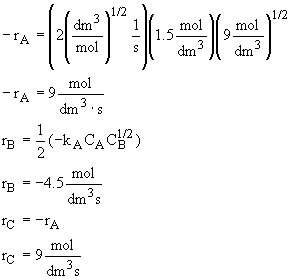What is the reaction rate law for the reaction ![]() if the reaction is elementary? What is rB? What is rC?
Calculate the rates of A, B, and C in a CSTR where the concentrations are CA
= 1.5 mol/dm3, CB = 9 mol/dm3 and kA
= 2 (dm3/mol)(1/2)(1/s).
if the reaction is elementary? What is rB? What is rC?
Calculate the rates of A, B, and C in a CSTR where the concentrations are CA
= 1.5 mol/dm3, CB = 9 mol/dm3 and kA
= 2 (dm3/mol)(1/2)(1/s).
|
![]()
Hint 2: What is rB?
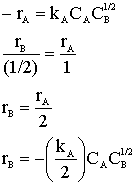
Hint 3: What is rC?
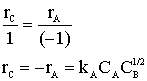
What is the reaction rate law for the reaction ![]() if the reaction is elementary? What is rB? What is rC?
Calculate the rates of A, B, and C in a CSTR where the concentrations are CA
= 1.5 mol/dm3, CB = 9 mol/dm3 and kA
= 2 (dm3/mol)(1/2)(1/s).
Solution
if the reaction is elementary? What is rB? What is rC?
Calculate the rates of A, B, and C in a CSTR where the concentrations are CA
= 1.5 mol/dm3, CB = 9 mol/dm3 and kA
= 2 (dm3/mol)(1/2)(1/s).
Solution
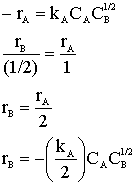
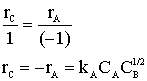
Let's calculate the rate if,
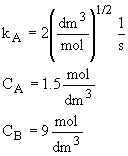
Then