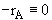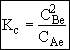Write the rate law for the elementary reaction
![]()
Here kfA and krA are the forward and reverse specific reaction rates both defined with respect to A.
Write the rate law for the elementary reaction
Here kfA and krA are the forward and reverse specific reaction rates both defined with respect to A.
Hint: What is the net rate of reaction?
|
|
What is the net rate of reaction?
(1) ![]()
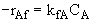
(2) ![]()
![]()
![]()
Solution
Write the rate law for the elementary reaction
![]()
Here kfA and krA are the forward and reverse specific reaction rates both defined with respect to A.
(1) 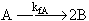
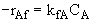
(2) 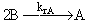
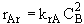

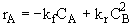
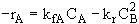
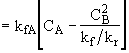
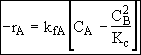
At equilibrium