Consider the liquid phase reaction

which is to take place in a PFR. The following data was obtained in a batch reactor.
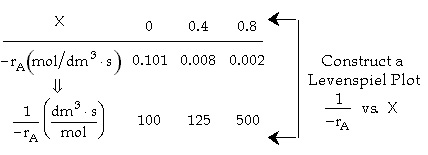
|
X |
0 |
0.4 |
0.8 |
| -ra(mol/dm3.s) | 0.01 | 0.008 |
0.002 |
If the molar feed of A to the PFR is 2 mol/s, what PFR volume is necessary
to achieve 80% conversion under identical conditions as those under which the batch data
was obtained?
Hint 1: What is the PFR design equation and how should the
data be plotted?
Hint 2: How can you evaluate the design equation?
Hint 1
FAo = 2 mol/s, fed to a plug flow reactor
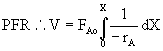
Thus, one needs (1/-rA) as a function of X
Hint 2
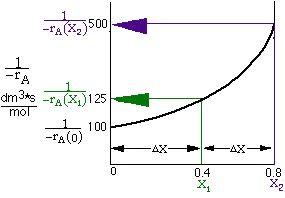
You can either take the area under the curve and multiply by FAO or you can
use one of the formulas in Appendix A.4.
Solution
FAo = 2 mol/s, fed to a plug flow reactor
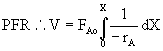
Thus, one needs (1/-rA) as a function of X


You can either take the area under the curve and multiply by FAO or you can use one of the formulas in Appendix A.4.
For Simpon's three point formula we have:
![]()
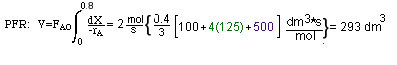
To reach 80% conversion, your PFR must be 293.3 dm3.