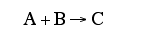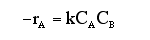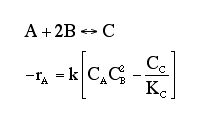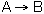
At a particular time t, the rate of formation of B in the reaction, rB, is 10 mole/dm3*min. Which of the following are true?
- The rate of disappearance of B is -10 moles/dm3*min.
- The rate of formation of A is -10 mole/dm3*min.
- The rate of disappearance of A is 10 moles/dm3*min.
- rA = -10 moles/dm3*min
- -rA = 10 moles/dm3*min
- -rB = -10 moles/dm3*min
- All of the above
- None of the above