3. Flocculation (Collision) Kinetics
The particle can also increase is size as a result of collision and subsequent coagulation with other particles. The rate of agglomeration of particles is
![]()
The rate of flocculation (collision) of the particles is proportional to the square of the concentration of particles is
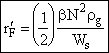 # of collisions/kg(gas)/s
# of collisions/kg(gas)/s
[0 < r’F < 5.9´1010] [Seinfeld, 1986] (15)
The 1/2 is included so that we don’t count the particle collisions twice. Where b is the collision coefficient and Ws a correction factor that can either increase or decrease the flocculation rate depending on the gas and particle properties.
The Flocculation Parameters
Again the parameter values for the APFR properties and operating conditions are given just above Figure 1.
b is the collision coefficient
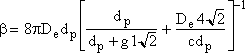 m3/s
m3/s
[5´10-16 < b < 7.2´10-15 m3/s]
dp is the particle diameter
De is the particle diffusion coefficient
![]() (m2/s)
(m2/s)
[1.5´10-10 < De < 8.5´10-4 m2/s]
c is the particle velocity
![]() (m/s), [0.0146 < c < 1408 m/s]
(m/s), [0.0146 < c < 1408 m/s]
rp is the density of the solid phase = 2700 kg/m3
vp is the particle
volume ![]() m3 [1.2´10-29
< vp < 5.2´10-20]
m3 [1.2´10-29
< vp < 5.2´10-20]
g1 is the transition parameter ![]() (m)
(m)
[1.35´10-8 < g1 < 1.53´10-6 m]
la is the mean
free path for the particle ![]() m
[2.6´10-8 < la
<1.5´10-6]
m
[2.6´10-8 < la
<1.5´10-6]
m
is the viscosity of the career gas ![]() kg/m/s
kg/m/s
[3.4´10-5 < m < 4.9´10-5 kg/m/s]
Ws is the stability factor that can either retard or accelerate flocculation.
1 < Ws < 106
We note Ws can be smaller than 1 for bipolarity charged aerosols.