Ozone Depletion
Web Module
Anjan K. Chakrabarti
Alejandra De Obeso
Dr. Nihat M. Gürmen
Prof. H. Scott Fogler
![]()
![]()
![]()
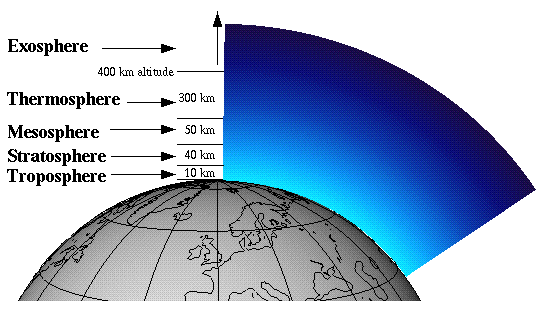
The atmosphere is split up into 5 distinct regions: the troposphere, the stratosphere, the mesosphere, the thermosphere, and the exosphere. The region of interest to us, with regard to the ozone hole, is the stratosphere. Ozone in the stratosphere is commonly referred to as “good ozone,” as opposed to ozone in the troposphere, which is given the name “bad ozone.” Ozone in the stratosphere absorbs harmful ultraviolet radiation to form atomic oxygen and molecular oxygen, thus keeping the UV radiation from reaching the surface of the Earth.
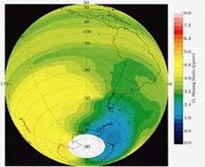
Image courtesy of Dr. Glenn Carver
http://www.atm.ch.cam.ac.uk/tour/The model detailed in this module will be using concentration data taken 30 km above the Earth’s surface, in the heart of the stratosphere. It is important to note that the ozone layer is not in the stratosphere, but determines the form of the stratosphere by creating an inversion layer which traps molecules in the troposphere. This inversion layer is a result of the increasing temperature and altitude that we see in the stratosphere as a result of the absorption of UV radiation.
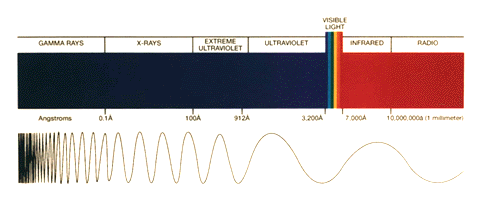
The amount of ultraviolet radiation that hits the surface of the Earth is directly proportional to the amount of ozone in the stratosphere. That is why maintaining a steady concentration of ozone in the stratosphere is important. Ultraviolet radiation can be broken down into UV-A, UV-B, and UV-C regions.
Image courtesy of Dr. Glenn Carver
http://www.atm.ch.cam.ac.uk/tour/
UV-A: This radiation has wavelengths between 320 and 380 nanometers. UV-A is hardly absorbed at all by the Earth's atmosphere, and it reaches its surface with nearly full intensity. UV-A radiation is used in tanning salons and fluorescent lamps, although it is believed that prolonged exposure can lead to detrimental health consequences.
UV-B: This radiation has wavelengths between 290 and 320 nanometers. UV-B radiation has been identified as hazardous, and is primarily responsible for sunburns. UV-B radiation is mostly absorbed by the ozone layer.
UV-C: Smaller wavelengths between 250 and 290 are designated as UV-C radiation. UV-C is extremely hazardous, and is often used as a sterilizing agent. UV-C is completely absorbed by the ozone layer, which is fortunate since humans have no natural defense against this kind of radiation.
The real concern here is UV-B radiation, which has the potential to penetrate the Earth’s surface in greater amounts if ozone depletion continues. Here are a few health effects of UV-B radiation:
Skin Cancer - The correlation between UV exposure and skin cancer has been established, but not characterized quantitatively. Basal and squamous cell cancers are the most common forms, but there is also the possibility of melanoma. Basal and squamous cancer cells are found in the form of tumors on the neck, hands, face, and arms. These three forms of skin cancer can further be explored on the Skin Cancer Foundation page.
Premature Aging of the Skin - UV radiation that penetrates into the skin can damage connective and supporting tissues, causing the skin’s texture to weaken and “age.” Those persons exposed to a large amount of UV-B radiation in their youth will find wrinkles, folds, and roughness in their skin 10-20 years before those who do not.
Cataracts and Retinal Damage - Researchers have found that there is a strong correlation between long-term exposure to UV radiation and cataracts, as well as the degeneration of the cornea. Permanent damage to the retina is also an effect of prolonged exposure to UV-B radiation. The eye can withstand a small amount of UV radiation, but a large dose can have very harmful effects on vision.
If more UV radiation is able to penetrate the Earth’s surface, more and more cases of skin cancer and eye damage will appear in the human population. Even more devastating is the thought that if enough ozone is depleted in the stratosphere, then UV-C radiation will be able to reach the Earth's surface and have disastrous effects on all organisms.
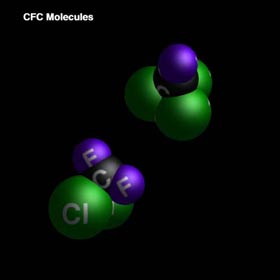
Chlorofluorocarbons (CFCs) are a major factor in ozone degradation. CFCs are used extensively in industrialized nations: they serve as refrigerants, blowing agents, and propellants in aerosol cans. These substances are attractive to industry because they are generally nontoxic and chemically inert.
CFCs are particularly dangerous to the ozone layer. Through photochemical dissociation, chlorine is released into the stratosphere causing the halogen catalyzed degradation of ozone, which will be studied further on.
See QuickTime Movie of Ozone Destruction due to chlorine released from CFCs
(Embedded, Direct)Decrease in the use of CFCs in industrial processes is a necessity for the survival of the ozone layer. The model used in this module will kinetically analyze how chlorine released from CFCs destroys a large number of ozone molecules before it is removed from the stratosphere, among other ozone-depleting agents and radicals.