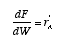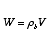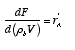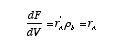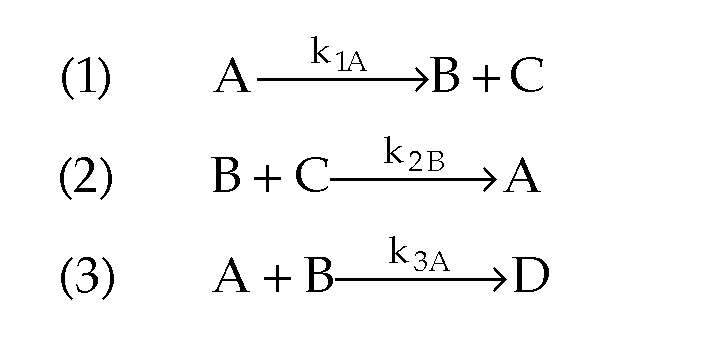- When accounting for pressure drop in a membrane reactor, does the
same method as we would use with a PFR apply?
Yes, 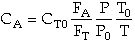
- Why can't you write the membrane equations in terms of conversion
X?
Because you can't relate the concentration of the product diffusing out, CB, and X.
- Would there ever by a case in which a membrane reactor would be
used for the reverse? I.e. if H2, for example, was used as
a reactant in a reaction, would one ever want to run concentrated H2
along the sides of the reactor and let it diffuse into the reaction
zone?
Yes, especially when O2 is one of the reactants.
- In a membrane reactor, how can one quantify the equilibrium point
on the graph of F vs. V?
In this region (i.e. after the knee) the reaction is in equilibrium
and the rate of removal of B is what limits the overall rate of reaction.
- How can you assume that a semi-batch system has constant density?
(p.199).
Most liquids do not change density during the course of the reaction.
- Do we only use the
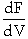 form of the PBR design equation for membrane reactors (IMRCF)?
form of the PBR design equation for membrane reactors (IMRCF)?
NO!! The mole balance is 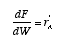
The relationship between reactor volume and catalyst weight is 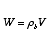
Substituting for W 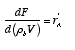
Then 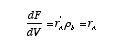
- Why do they use a batch reactor to determine k if they are going
to be using CSTR in actual industrial process?
Batch experiments are most always easier to take the necessary data to determine
k.
- In what cases would you use the order of magnitude reaction times
other than to check k values that you calculate?
When you are short on time and want to get quick engineering estimates.
- At some of the polymer plants and refineries I've visited, a huge
problem is fouling of the reactors. The plant workers would sometimes
have to go into the reactors to break through the solids/sludge that
adhere to the reactor walls. I imagine this solid build up leads to
a drastic volume decrease. So, how do we take into account the change
of volume and it's detrimental effect to conversion?
Good point. It would change the volume; however, catalyst decay by fouling is usually
more important. See Ch.10.
- Is rinsing the reactor with water after a batch ample cleaning,
or are chemical cleans necessary in between batches?
It depends, if there are no side reactions, a chemical clean is probably
not necessary. Also the larger the reactor the greater the cleaning
time.
- When do you need to consider overall selectivity (or yield)? What is
the advantage of having the two different kinds of selectivity (or yield)?
The instantaneous selective guides our initial reactor selection. For
a CSTR the instantaneous selectivity is the same as overall selectivity.
- What is the purpose of the instantaneous selectivity parameter? It
seems to be just a descriptive term?
No, it helps to synthesize reaction schemes (see p.285-298).
- Why do you use a CSTR if you want to keep CA low and a
PFR when you want it high? This refers specifically to the home problem?
CSTRs operate at the lowest concentration. Feed drops to the low concentration
immediately upon entry. PFR concentration starts high at entrance and drops
slowly as one moves down the reactor.
- I'm confused about space time. Is it characteristic of a reactor
(space time = overall volume/ volumetric flow rate), which I know to be
the case when the volume is fixed, but is it the case for PFR/PBR? Or,
for flow reactors, does it mark the position in the reactor just like V
does (space time = current volume in the reactor/ volumetric flow rate)?
I see plots of parameters (e.g. X, FA) vs. space time, I am
confused whether you're talking about different reactors (giving different
space times) or different positions in the same reactor. Please see
Figures E6-6.1 and E6-7.1
For a CSTR it is always the total volume divided by the volumetric flow
rate. Either the flow rate or total reactor volume may be varied to vary
t shown in Fig. E6-7.1. For a PFR space time can be related to the
location down the reactor but it also represents the total volume divided
by the volumetric flow rate where either one or the other is varied to
vary the space time t.
- What happens when you have multiple reactions that are reversible?

Just treat as:
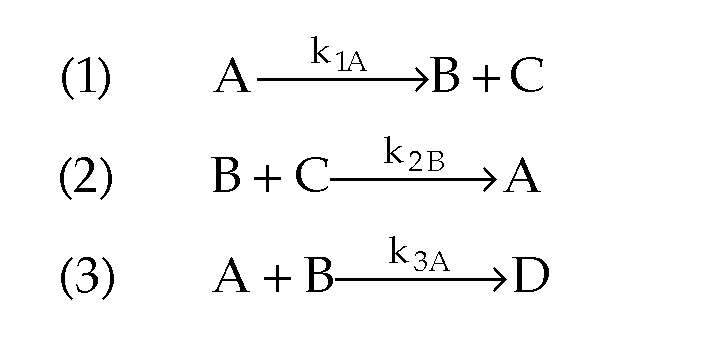
When there are parallel reactions, the book discusses many cases
comparing the reaction orders of the equations. What kind of reactor should
be used if the two reactions have the same order (a1
= a2)?
In this case you need to work with temperature dependence to affect
selectivity.
- In chapter 6, we consider the multiple reactions under isothermal
conditions, and we will cover in later chapters non-isothermal multiple
reactions. Is temperature the only main concern in the design for maximizing
selectivity?
We can vary inlet conditions and type of reactors
- Can catalysts be used in a competing reaction scenario? Can they
be used to give more of a preferred product?
Yes. Definitely!
![]()