One must take ratio of the two! Usually when (UA/mCP) < 0.1, you can expand exponents in a Taylor series to get Q = UA(Ta - T).
Good point!! Yes it does.

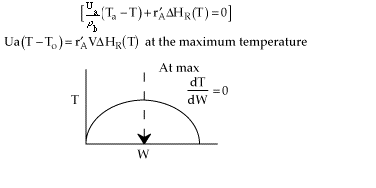
No! The term dT/dW will only equal zero if there is a maximum
in the temperature down the length (i.e. W) of the packed bed. For a single
reaction, the maximum will only occur if the reaction is exothermic and
there is heat exchange.

then
A lower activation energy means the reaction is less temperature sensitive. The G(t) curve also depends on t.
It simply shifts the R(t) curve. It is easily included in the heat removed
term
