There are tables in the literature. For a first-order gas phase reaction, an order of magnitude value is A=1013s-1. Generally the frequency factor is independent of temperatures, however on occasion it can be a weak function of temperature. See p.944
If the limiting reactant is not chosen as the basis of calculations, one could calculate a NEGATIVE concentration. See Example 3-5 (p.90).
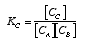
and the k in the rate laws?
KC is an equilibrium constant, and k is specific rate constant and has units of time. The concentration equilibrium constant KC does not.
ONLY in very very rare instances at very high pressures such as, 6000 atm is k a function of pressure. See p.220 and CD-ROM on "Critiquing what you read."
Arrhenius Equation is k = Ae-E/RT
The frequency factor, A, is the coefficient of the exponential term.
It has the same units as k.
It is related to the number of collisions between molecules.
See p.942 and 943.
One can classify reactions by their overall order of reaction.
These can be found in the literature, journals, books, tables, etc., see the footnote on p.75. They can also be determined in the laboratory. See Ch.5
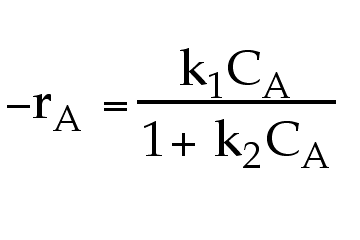 determined as a function of concentration and how
do we find the rate law of a chemical reaction if this is not elementary?
determined as a function of concentration and how
do we find the rate law of a chemical reaction if this is not elementary?
The rate law is determined only from experiment, see chapter 5. Also see chapter 7 for a discussion of non-elementary reactions.
It is a function of concentration of species participating in the reaction and whether or not there is a catalyst.
So that -rA has always the same units (mol/dm3 s), no matter what the reaction order.
The activation energy is a constant for each reaction (at least in a range of temperatures). It is related to the strength of the chemical bonds that must be broken and formed for the chemical reaction to take place.
For example, if the rate law is 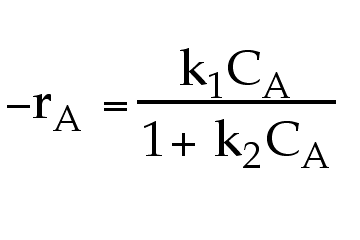 we can see that for large values of k2CA ( k2CA >> 1) the rate law becomes
we can see that for large values of k2CA ( k2CA >> 1) the rate law becomes 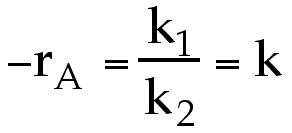 , i.e. apparant zero order. If a zero-order reaction is conducted in a batch reactor, the concentration is a linear function of time (i.e. dC/dt = k and CA= CAo - kt).
, i.e. apparant zero order. If a zero-order reaction is conducted in a batch reactor, the concentration is a linear function of time (i.e. dC/dt = k and CA= CAo - kt).