The CSTR is always operating a the lowest concentration, the exit concentration. When say two CSTRs are in series, the first operates at a higher concentration, therefore the rate is greater, therefore the conversion is greater. The second reactor in series builds on the conversion in the first reactor. The conversion in the parallel scheme is the same as the conversion to the first reactor to the series scheme. See Example 5-2.
The PBRs in parallel are ued when there would otherwise be a large pressure drop in one long reactor or identically several PBRs connected in series.
You can have a pressure drop in liquid phase systems, but it does not affect the reaction rate because liquids are virtually incompressible and therefore the concentration does not change with pressure.
Not for a CSTR, only a PFR/PBR when there is a significant pressure drop.
- How is the Damköhler (Da) number defined for a reaction
(A + B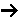 C) when the reaction is first order in A, first order in B, but
second order overall?
C) when the reaction is first order in A, first order in B, but
second order overall?
Just substitute the rate law evaluated entrance to the reactor, -rA0, [e.g. -rAo=kCAoCBo] into the definition
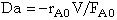 .
.
- Is Da always indicative of certain conversion?
Yes, for irreversible reactions.
- How does defining an extra variable, the Damk�hler number,
save us time and confusion, as opposed to solving without it? When
should it be used?
It serves as rule of thumb. When Da < 0.1 then X < 10% and when Da >10 then X >90%.
The onstream time for flow systems is much much greater than the down time for cleaning and repair.
![]()
Neglecting ![]() X
X
![]()
For what values of ![]() X
is this valid?
X
is this valid?
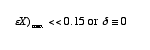
The same is true for a PFR![]() If you increase
If you increase ![]() and you increase X.
and you increase X.
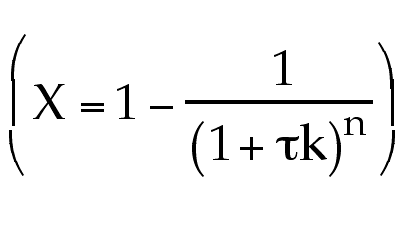 always the same no matter the order
of reaction?
always the same no matter the order
of reaction?
No! This equation is only for first order.